Diacylglycerol O-acyltransferase 2, a Novel Target of Flavivirus NS2B3 Protease, Promotes Zika Virus Replication by Regulating Lipid Droplet Formation
- PMID: 39449854
- PMCID: PMC11499588
- DOI: 10.34133/research.0511
Diacylglycerol O-acyltransferase 2, a Novel Target of Flavivirus NS2B3 Protease, Promotes Zika Virus Replication by Regulating Lipid Droplet Formation
Abstract
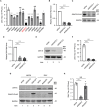
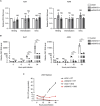
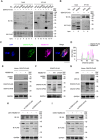
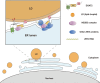
Various lipid metabolism-related factors are essential for Zika virus (ZIKV) replication. In this study, we revealed a crucial role of diacylglycerol O-acyltransferase 2 (DGAT2) in ZIKV replication using a short hairpin RNA-based gene knockdown technique. The replication of ZIKV was significantly inhibited by DGAT2 depletion in multiple cell lines and restored by trans-complementation with DGAT2. Mechanistically, DGAT2 is recruited in the viral replication complex by interacting with non-structural (NS) proteins. Among them, both human and murine DGAT2s can be cleaved by NS2B3 at the 122R-R-S124 site. Interestingly, the cleavage product of DGAT2 becomes more stable and is sufficient to promote the lipid droplet (LD) formation independent of its enzymatic activity. This work identifies DGAT2 as a novel target of the viral protease NS2B3 and elucidates that DGAT2 is recruited by viral proteins into the replication complex, thereby playing a proviral role by promoting LD formation, which advances our understanding of host-flavivirus interaction.
Copyright © 2024 Xiaotong Luo et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects--Reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

