A systematic review and meta-analysis of the efficacy and safety of iguratimod in the treatment of inflammatory arthritis and degenerative arthritis
- PMID: 39449973
- PMCID: PMC11499590
- DOI: 10.3389/fphar.2024.1440584
A systematic review and meta-analysis of the efficacy and safety of iguratimod in the treatment of inflammatory arthritis and degenerative arthritis
Abstract
Objective: To assess the efficacy and safety of iguratimod (IGU) in the treatment of inflammatory arthritis and degenerative arthritis.
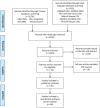
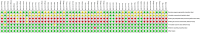
Methods: Initially, randomized controlled trials (RCTs) on using IGU in treating inflammatory arthritis and degenerative arthritis were systematically gathered from various databases up to February 2024. Subsequently, two researchers independently screened the literature, extracted data, assessed the risk of bias in included studies, and conducted a meta-analysis using RevMan 5.4 software.
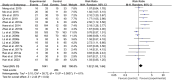
Results: Fifty-four RCTs involving three inflammatory arthritis were included, including ankylosing spondylitis (AS), osteoarthritis (OA), and rheumatoid arthritis (RA). For AS, the meta-analysis results showed that IGU may decrease BASDAI (SMD -1.68 [-2.32, -1.03], P < 0.00001) and BASFI (WMD -1.29 [-1.47, -1.11], P < 0.00001); IGU may also decrease inflammatory factor [ESR: (WMD -10.33 [-14.96, -5.70], P < 0.0001); CRP: (WMD -10.11 [-14.55, -5.66], P < 0.00001); TNF-α: (WMD -6.22 [-7.97, -4.47], P < 0.00001)]. For OA, the meta-analysis results showed that IGU may decrease VAS (WMD -2.20 [-2.38, -2.01], P < 0.00001) and WOMAC (WMD -7.27 [-12.31, -2.24], P = 0.005); IGU may also decrease IL-6 (WMD -8.72 [-10.00, -7.45], P < 0.00001). For RA, the meta-analysis results showed that IGU may improve RA remission rate [ACR20: (RR 1.18 [1.02, 1.35], P = 0.02); ACR50: (RR 1.32 [1.05, 1.64], P = 0.02); ACR70: (RR 1.44 [1.02, 2.04], P = 0.04)] and decrease DAS28 (WMD -0.92 [-1.20, -0.63], P < 0.00001); IGU may also decrease inflammatory factors [CRP: (SMD -1.36 [-1.75, -0.96], P < 0.00001); ESR: (WMD -9.09 [-11.80, -6.38], P < 0.00001); RF: (SMD -1.21 [-1.69, -0.73], P < 0.00001)]. Regarding safety, adding IGU will not increase the incidence of adverse events.
Conclusion: IGU might emerge as a promising and secure therapeutic modality for addressing AS, OA, and RA.
Systematic review registration: Identifier PROSPERO: CRD42021289249.
Keywords: degenerative arthritis; iguratimod; inflammatory arthritis; meta-analysis; systematic review.
Copyright © 2024 Long, Zeng, Yang, Chen, Luo, Dai, He, Deng, Ge, Zhu, Hao and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



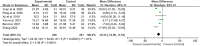
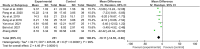
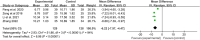

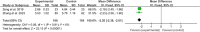
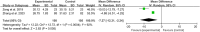









References
-
- Bai Y. J., Wang X. Y., Yao Y. J. (2021). Observation on the clinical effect of iguratimod in the treatment of axial spondyloarthritis. Chin. Med. Innov. 18 (2), 44–47. 10.3969/j.issn.1674-4985.2021.02.011 - DOI
-
- Bi W. H. (2019). The effect of Iguratimod combined with methotrexate on serum VEGF levels in patients with rheumatoid arthritis and evaluation of the efficacy[D]. Inner Mongolia Medical University.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

