Pharmacovigilance: the evolution of drug safety monitoring
- PMID: 39450127
- PMCID: PMC11500540
- DOI: 10.1080/20523211.2024.2417399
Pharmacovigilance: the evolution of drug safety monitoring
Abstract
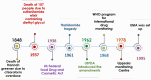
The Pharmacovigilance system is aimed to promote and protect public health by ensuring the availability of essential medicines in the market and reducing the burden of ADRs. Pharmacovigilance is derived from two words; pharamakon rooted in the Greek word that means medicinal substance and vigilia rooted in the Latin word to keep watch. This concept evolved after Hannah Greener died in 1848 after having a tonsillectomy with chloroform. As a result of the Thalidomide tragedy, drug regulation in Europe has forever changed. From its earliest beginnings to its current state, pharmacovigilance has been shaped by several major milestones. The historical phases of pharmacovigilance can help us understand the value of pharmacovigilance and identify the challenges that lie ahead. Despite advancements in technology, it is imperative that we continue to strive for excellence in pharmacovigilance to ensure all individuals' safety and health. Through collaboration between the Council for International Organizations of Medical Sciences (CIOMS), World Health Organization (WHO), and the International Conference on Harmonization (ICH), Pharmacovigilance has evolved into a regulatory activity.
Keywords: Adverse drug reactions; drug safety; medicines safety; monitoring; pharmacovigilance; public health.
© 2024 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
CIOMS and ICH initiatives in pharmacovigilance and risk management: overview and implications.Drug Saf. 2004;27(8):509-17. doi: 10.2165/00002018-200427080-00004. Drug Saf. 2004. PMID: 15154824 Review.
-
Pharmacovigilance and Biomedical Informatics: A Model for Future Development.Clin Ther. 2016 Dec;38(12):2514-2525. doi: 10.1016/j.clinthera.2016.11.006. Epub 2016 Nov 29. Clin Ther. 2016. PMID: 27913029 Review.
-
Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance.Drug Saf. 2011 Mar 1;34(3):187-97. doi: 10.2165/11586620-000000000-00000. Drug Saf. 2011. PMID: 21332243 Review.
-
Pharmacovigilance during the pre-approval phases: an evolving pharmaceutical industry model in response to ICH E2E, CIOMS VI, FDA and EMEA/CHMP risk-management guidelines.Drug Saf. 2006;29(8):657-73. doi: 10.2165/00002018-200629080-00003. Drug Saf. 2006. PMID: 16872240 Review.
-
Pharmacovigilance Discussion Forum--The European Generic Medicines Association's 8th Annual Meeting (January 21, 2015--London, UK).Drugs Today (Barc). 2015 Jan;51(1):89-92. doi: 10.1358/dot.2015.51.1.2285587. Drugs Today (Barc). 2015. PMID: 25685861
Cited by
-
Empowering Patient Safety: Assessment of Adverse Drug Reaction Knowledge and Practice Among Pharmacy Professionals.Pharmacy (Basel). 2024 Dec 29;13(1):1. doi: 10.3390/pharmacy13010001. Pharmacy (Basel). 2024. PMID: 39846624 Free PMC article.
-
Precision medication based on the evaluation of drug metabolizing enzyme and transporter functions.Precis Clin Med. 2025 Feb 22;8(1):pbaf004. doi: 10.1093/pcmedi/pbaf004. eCollection 2025 Mar. Precis Clin Med. 2025. PMID: 40110576 Free PMC article. Review.
-
Oropharyngeal adverse drug reactions: knowledge, attitudes, and practice (KAP) among Italian healthcare professionals and students.Front Public Health. 2025 Apr 11;13:1572611. doi: 10.3389/fpubh.2025.1572611. eCollection 2025. Front Public Health. 2025. PMID: 40290502 Free PMC article.
References
-
- Bahri, P., & Arlett, P. (2014). Regulatory pharmacovigilance in the European Union. In Andrews E. B. & Moore N. (Eds.), Mann’s pharmacovigilance (pp. 171–184). Wiley. 10.1002/9781118820186.ch13a - DOI
-
- Cui, X., Wang, L.-X., Liu, G.-Y., & Xie, Y.-M. (2021). Enlightenment of international pharmacovigilance system on establishment of pharmacovigilance system of Chinese medicine. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 46(21), 5450–5455. 10.19540/j.cnki.cjcmm.20210918.501 - DOI - PubMed
-
- EU . (2008). European Commission. MEMO/08/782: Strengthening pharmacovigilance to reduce adverse effects of medicines. https://ec.europa.eu/commission/presscorner/detail/en/memo_08_782.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials