Impact of cooperative or competitive dynamics between the yeast Saccharomyces cerevisiae and lactobacilli on the immune response of the host
- PMID: 39450162
- PMCID: PMC11499123
- DOI: 10.3389/fimmu.2024.1399842
Impact of cooperative or competitive dynamics between the yeast Saccharomyces cerevisiae and lactobacilli on the immune response of the host
Abstract
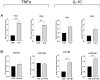
Fungi and bacteria can be found coexisting in a wide variety of environments. The combination of their physical and molecular interactions can result in a broad range of outcomes for each partner, from competition to cooperative relationships. Most of these interactions can also be found in the human gastrointestinal tract. The gut microbiota is essential for humans, helping the assimilation of food components as well as the prevention of pathogen invasions through host immune system modulation and the production of beneficial metabolites such as short-chain fatty acids (SCFAs). Several factors, including changes in diet habits due to the progressive Westernization of the lifestyle, are linked to the onset of dysbiosis statuses that impair the correct balance of the gut environment. It is therefore crucial to explore the interactions between commensal and diet-derived microorganisms and their influence on host health. Investigating these interactions through co-cultures between human- and fermented food-derived lactobacilli and yeasts led us to understand how the strains' growth yield and their metabolic products rely on the nature and concentration of the species involved, producing either cooperative or competitive dynamics. Moreover, single cultures of yeasts and lactobacilli proved to be ideal candidates for developing immune-enhancing products, given their ability to induce trained immunity in blood-derived human monocytes in vitro. Conversely, co-cultures as well as mixtures of yeasts and lactobacilli have been shown to induce an anti-inflammatory response on the same immune cells in terms of cytokine profiles and activation surface markers, opening new possibilities in the design of probiotic and dietary therapies.
Keywords: Saccharomyces cerevisiae; fermented food; host immune system modulation; lactobacilli; microbial ecology; short-chain fatty acids; trained immunity; yeasts.
Copyright © 2024 Nenciarini, Rivero, Ciccione, Amoriello, Cerasuolo, Pallecchi, Bartolucci, Ballerini and Cavalieri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
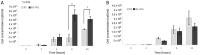

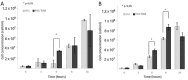
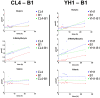
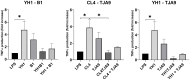


References
-
- Behzadi P, Dodero VI, Golubnitschaja O. Systemic inflammation as the health-related communication tool between the human host and gut microbiota in the framework of predictive, preventive, and personalized medicine. In: All Around Suboptimal Health: Advanced Approaches by Predictive, Preventive and Personalised Medicine for Healthy Populations. Springer Nature Switzerland, Cham: (2024). p. 203–41.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

