Bispecific antibody targets and therapies in multiple myeloma
- PMID: 39450163
- PMCID: PMC11499143
- DOI: 10.3389/fimmu.2024.1424925
Bispecific antibody targets and therapies in multiple myeloma
Abstract
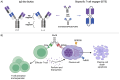
Recently, several bispecific antibodies (BsAbs) have been approved for the treatment of relapsed multiple myeloma (MM) after early phase trials in heavily pre-treated patients demonstrated high response rates and impressive progression-free survival with monotherapy. These BsAbs provide crucial treatment options for relapsed patients and challenging decisions for clinicians. Evidence on the optimal patient population, treatment sequence, and duration of these therapeutics is unknown and subject to active investigation. While rates of cytokine release syndrome and neurotoxicity appear to be lower with BsAbs than with CAR T-cells, morbidity from infection is high and novel pathways of treatment resistance arise from the longitudinal selection pressure of chronic BsAb therapy. Lastly, a wealth of novel T-cell engagers with unique antibody-structures and antigenic targets are under active investigation with promising early outcome data. In this review, we examine the mechanism of action, therapeutic targets, combinational approaches, sequencing and mechanisms of disease relapse for BsAbs in MM.
Keywords: T-cell engagers; bispecific antibodies; combination (combined) therapy; immunotherapy; multiple myeloma; sequencing; treatment resistance.
Copyright © 2024 Rees, Abdallah, Yohannan and Gonsalves.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Mateos M-V, Weisel K, De Stefano V, Goldschmidt H, Delforge M, Mohty M, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. (2022) 36:1371–6. doi: 10.1038/s41375-022-01531-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

