Intranasal sensitization model of alopecia areata using pertussis toxin as adjuvant
- PMID: 39450167
- PMCID: PMC11499204
- DOI: 10.3389/fimmu.2024.1469424
Intranasal sensitization model of alopecia areata using pertussis toxin as adjuvant
Abstract
Background: Nasopharyngeal Bordetella pertussis (BP) colonization is common, with about 5% of individuals having PCR evidence of subclinical BP infection on nasal swab, even in countries with high vaccination rates. BP secretes pertussis toxin (PTx). PTx is an adjuvant commonly used to induce autoimmunity in multiple animal models of human disease. Colocalization of PTx and myelin from myelinated nerves in the nasopharynx may lead to host sensitization to myelin with subsequent autoimmune pathology.
Methods: C57BL/6J female adult mice were given varied doses and schedules of intranasal PTx, MOG35-55 antigen, or controls to test whether intranasal administration of PTx and myelin oligodendrocyte peptide (MOG35-55) could induce experimental autoimmune encephalomyelitis (EAE) in mice. While we observed systemic cell-mediated immunity against MOG35-55, we did not observe EAE. Unexpectedly, many mice developed alopecia. We systematically investigated this finding.
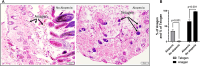
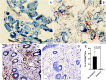
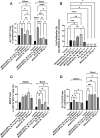
Results: Patchy alopecia developed in 36.4% of mice with the optimized protocol. Pathology consistent with alopecia areata was confirmed histologically by documenting concomitant reduced anagen phase and increased telogen phase hair follicles (HFs) in biopsies from patches of hair loss in mice with alopecia. We also found reduced CD200 staining and increased CD3+T cells surrounding the HFs of mice with alopecia compared to the mice without alopecia, indicating HF Immune Privilege (HFIP) collapse. Systemic immune responses were also found, with increased proportions of activated T cells and B cells, as well as MHCII+ dendritic cells in peripheral blood and/or splenocytes. Finally, in mice initially exposed to intranasal MOG35-55 and PTx in combination, but not to either agent alone, splenocytes were shown to proliferate after in vitro stimulation by MOG35-55. Consistent with prior investigations, PTx exhibited a dose-response effect on immune cell induction and phenotype, with the lowest PTx dose failing to induce autoimmunity, the highest PTx dose suppressing autoimmunity, and intermediate doses optimizing autoimmunity.
Conclusions: We propose that this is the first report of an autoimmune disease in an animal model triggered by colocalization of intranasal PTx and autoantigen. This model parallels a natural exposure and potential intranasal sensitization-to-pathology paradigm and supports the plausibility that nasopharyngeal subclinical BP colonization is a cause of alopecia areata.
Keywords: Bordetella pertussis; autoantigen; autoimmunity; immunization; myelin oligodendrocyte peptide; nasopharyngeal colonization.
Copyright © 2024 Liu, Freeborn, Okeugo, Armbrister, Saleh, Fadhel Alvarez, Hoang, Park, Lindsey, Rapini, Glazer, Rubin and Rhoads.
Conflict of interest statement
KR and SG are employed by ILiAD Biotechnologies LLC, which is developing a vaccine for the prevention of Bordetella pertussis infection. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author YL declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision. The authors declare that this study received funding from ILiAD Biotechnologies LLC (P00427002 to JR). The funder had the following involvement in the study: the study design and the writing of this article (review and edit).
Figures

References
-
- Rubin K, Glazer S. Potential role of bordetella pertussis in celiac disease. Int J Celiac Dis. (2015) 3:75–6. doi: 10.12691/ijcd-3-2-2 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

