Targeting STING signaling for the optimal cancer immunotherapy
- PMID: 39450170
- PMCID: PMC11500076
- DOI: 10.3389/fimmu.2024.1482738
Targeting STING signaling for the optimal cancer immunotherapy
Abstract
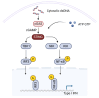
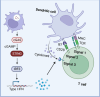
Despite the transformative impact of anti-PD-1/PD-L1 therapies, challenges such as low response rates persist. The stimulator of interferon genes (STING) pathway, a crucial element of innate immunity, emerges as a strategic target to overcome these limitations. Understanding its multifaceted functions in cancer, including antigen presentation and response to DNA damage, provides valuable insights. STING agonists, categorized into cyclic dinucleotides (CDNs) and non-CDNs, exhibit promising safety and efficacy profiles. Innovative delivery systems, including antibody-drug conjugates, nanocarriers, and exosome-based therapies, address challenges associated with systemic administration and enhance targeted tumor delivery. Personalized vaccines, such as DT-Exo-STING, showcase the adaptability of STING agonists for individualized treatment. These advancements not only offer new prospects for combination therapies but also pave the way for overcoming resistance mechanisms. This review focuses on the potential of targeting STING pathway to enhance cancer immunotherapy. The integration of STING agonists into cancer immunotherapy holds promise for more effective, personalized, and successful approaches against malignancies, presenting a beacon of hope for the future of cancer treatment.
Keywords: PD-1; PD-L1; STING; cancer immunotherapy; the tumor microenvironment.
Copyright © 2024 Xu and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

