Transition from acute kidney injury to chronic kidney disease in a long-term murine model of Shiga toxin-induced hemolytic-uremic syndrome
- PMID: 39450175
- PMCID: PMC11499141
- DOI: 10.3389/fimmu.2024.1469353
Transition from acute kidney injury to chronic kidney disease in a long-term murine model of Shiga toxin-induced hemolytic-uremic syndrome
Abstract
Introduction: Up to 40% of patients with typical hemolytic-uremic syndrome (HUS), characterized by microangiopathic hemolytic anemia and acute kidney injury (AKI), develop long-term consequences, most prominently chronic kidney disease (CKD). The transition from AKI to CKD, particularly in the context of HUS, is not yet fully understood. The objective of this study was to establish and characterize a Shiga toxin (Stx)-induced long-term HUS model to facilitate the study of mechanisms underlying the AKI-to-CKD transition.
Methods: C57BL/6J mice were subjected to 5, 10, 15, or 20 ng/kg Stx on days 0, 3, and 6 of the experiment and were sacrificed on day 14 or day 21 to identify the critical time of turnover from the acute to the chronic state of HUS disease.
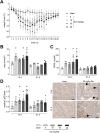
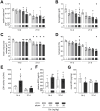
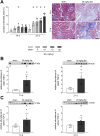
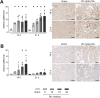
Results: Acute disease, indicated by weight loss, plasma neutrophil gelatinase-associated lipocalin (NGAL) and urea, and renal neutrophils, diminished after 14 days and returned to sham level after 21 days. HUS-associated hemolytic anemia transitioned to non-hemolytic microcytic anemia along with unchanged erythropoietin levels after 21 days. Renal cytokine levels indicated a shift towards pro-fibrotic signaling, and interstitial fibrosis developed concentration-dependently after 21 days. While Stx induced the intrarenal invasion of pro-inflammatory M1 and pro-fibrotic M2 macrophages after 14 days, pro-fibrotic M2 macrophages were the dominant phenotype after 21 days.
Conclusion: In conclusion, we established and characterized the first Stx-induced long-term model of HUS. This tool facilitates the study of underlying mechanisms in the early AKI-to-CKD transition following HUS and allows the testing of compounds that may protect patients with AKI from developing subsequent CKD.
Keywords: acute kidney injury; anemia; animal model; chronic kidney disease; fibrosis; hemolytic-uremic syndrome.
Copyright © 2024 Wegener, Dennhardt, Loeffler and Coldewey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

