Nanocarrier foliar uptake pathways affect delivery of active agents and plant physiological response
- PMID: 39450293
- PMCID: PMC11494269
- DOI: 10.1039/d4en00547c
Nanocarrier foliar uptake pathways affect delivery of active agents and plant physiological response
Abstract
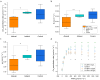
Layered double hydroxide (LDH) nanoparticles enable foliar delivery of genetic material, herbicides, and nutrients to promote plant growth and yield. Understanding the foliar uptake route of nanoparticles is needed to maximize their effectiveness and avoid unwanted negative effects. In this study, we investigated how delivering layered double hydroxide (d = 37 ± 1.5 nm) through the adaxial (upper) or abaxial (lower) side of leaves affects particle uptake, nutrient delivery, and photosynthesis in tomato plants. LDH applied on the adaxial side was embedded in the cuticle and accumulated at the anticlinal pegs between epidermal cells. On the abaxial side, LDH particles penetrated the cuticle less, but the presence of the stomata enables penetration to deeper leaf layers. Accordingly, the average penetration levels of LDH relative to the cuticle were 2.47 ± 0.07, 1.25 ± 0.13, and 0.75 ± 0.1 μm for adaxial, abaxial with stomata, and abaxial without stomata leaf segments, respectively. In addition, the colocalization of LDH with the cuticle was ∼2.3 times lower for the adaxial application, indicating the ability to penetrate the cuticle. Despite the low adaxial stomata density, LDH-mediated delivery of magnesium (Mg) from leaves to roots was 46% higher for the adaxial than abaxial application. In addition, adaxial application leads to ∼24% higher leaf CO2 assimilation rate and higher biomass accumulation. The lower efficiency from the abaxial side was, at least partially, a result of interference with the stomata functionality which reduced stomatal conductance and evapotranspiration by 28% and 25%, respectively, limiting plant photosynthesis. This study elucidates how foliar delivery pathways through different sides of the leaves affect their ability to deliver active agents into plants and consequently affect the plants' physiological response. That knowledge enables a more efficient use of nanocarriers for agricultural applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
-
- Hong J. Wang C. Wagner D. C. Gardea-Torresdey J. L. He F. Rico C. M. Foliar Application of Nanoparticles: Mechanisms of Absorption, Transfer, and Multiple Impacts. Environ. Sci.: Nano. 2021;8:1196–1210. doi: 10.1039/D0EN01129K. - DOI
-
- Takeshita V. de Sousa B. T. Preisler A. C. Carvalho L. B. do E. S. Pereira A. Tornisielo V. L. Dalazen G. Oliveira H. C. Fraceto L. F. Foliar Absorption and Field Herbicidal Studies of Atrazine-Loaded Polymeric Nanoparticles. J. Hazard. Mater. 2021;418:126350. doi: 10.1016/j.jhazmat.2021.126350. - DOI - PubMed
-
- Dan N. Transport and Release in Nano-Carriers for Food Applications. J. Food Eng. 2016;175:136–144. doi: 10.1016/j.jfoodeng.2015.12.017. - DOI
LinkOut - more resources
Full Text Sources
