Synaptic and extrasynaptic distribution of NMDA receptors in the cortex of Alzheimer's disease patients
- PMID: 39450669
- PMCID: PMC11667538
- DOI: 10.1002/alz.14125
Synaptic and extrasynaptic distribution of NMDA receptors in the cortex of Alzheimer's disease patients
Abstract
Background: Synaptic and extrasynaptic distribution of N-methyl-D-aspartate receptors (NMDARs) has not been addressed in the brain from Alzheimer´s disease (AD) subjects, despite their contribution to neurodegeneration.
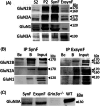
Methods: We have developed a protocol to isolate synaptic and extrasynaptic membranes from controls and AD frontal cortex. We characterized the distribution of the NMDAR subunits GluN2B, GluN2A, GluN1, and GluN3A, as well as post-translational modifications, such as phosphorylation and glycosylation.
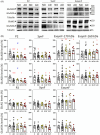
Results: Lower levels of synaptic GluN2B and GluN2A were found in AD fractions, while extrasynaptic GluN2B and GluN1 levels were significantly higher; GluN3A distribution remained unaffected in AD. We also identified different glycoforms of GluN2B and GluN2A in extrasynaptic membranes. Synaptic Tyr1472 GluN2B phosphorylation was significantly lower in AD fractions.
Discussion: Reduction of synaptic NMDAR subunits, particularly for GluN2B, is likely to contribute to synaptic transmission failure in AD. Additionally, the increment of extrasynaptic NMDAR subunits could favor the activation of excitotoxicity in AD.
Highlights: New protocol to isolate synaptic and extrasynaptic membranes from the human cortex. Low GluN2B and GluN2A levels in Alzheimer´s disease (AD) synaptic membranes. High GluN2B and GluN1 levels in AD extrasynaptic membranes. Specific glycoforms of extrasynaptic GluN2B and GluN2A. Low phosphorylation at Tyr1472 in synaptic GluN2B in AD.
Keywords: Alzheimer´s disease; GluN1; GluN2A; GluN2B; GluN3A; NMDA; Tyr1336; Tyr1472; extrasynaptic; human.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Figures



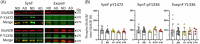


References
MeSH terms
Substances
Grants and funding
- PI22/01329/Fondo de Investigaciones Sanitarias, co-funded by the Fondo Europeo de Desarrollo Regional, FEDER "Investing in your future
- AICO/2021/308/Direcció General de Ciència i Investigació, Generalitat Valenciana
- CEX2021-001165-S/Centro de Excelencia Severo Ochoa, Agencia Estatal de Investigación
- Instituto de Investigación Sanitaria y Biomédica de Alicante (Isabial)
- INVEST-2023-157/Programa Investigo, Conselleria de Innovación, Universidades, Investigación y Sociedad Digital, , Generalitat Valenciana
LinkOut - more resources
Full Text Sources
Medical

