Stent Underexpansion Is an Underestimated Cause of Intrastent Restenosis: Insights From RESTO Registry
- PMID: 39450717
- PMCID: PMC11935687
- DOI: 10.1161/JAHA.124.036065
Stent Underexpansion Is an Underestimated Cause of Intrastent Restenosis: Insights From RESTO Registry
Abstract
Background: Despite improvement in devices, in-stent restenosis remains a frequent and challenging complication of percutaneous coronary interventions.
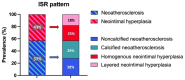
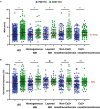
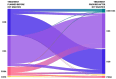
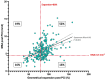
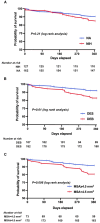
Methods and results: The RESTO (Morphological Parameters of In-Stent Restenosis Assessed and Identified by OCT [Optical Coherence Tomography]; study NCT04268875) was a prospective multicenter registry including patients presenting with coronary syndromes related to in-stent restenosis. All patients underwent preintervention OCT analysis, which led to analysis of in-stent restenosis phenotype, number of strut layers, and presence of stent underexpansion. The primary end point was the in-stent restenosis type according to the OCT morphological classification. The 1-year incidence of target vessel failure (a composite of death from cardiac causes, target-vessel myocardial infarction, or ischemia-driven target-vessel revascularization) was assessed. The study included 297 patients. The culprit stent was a drug-eluting stent in 74.2% of cases. OCT analysis revealed the presence of neoatherosclerosis in 57% (52% calcified), neointimal hyperplasia in 43% (58% homogeneous), stent underexpansion (minimal stent area <4.5 mm2) in 43%, and multiple stent layers in 30%. The prepercutaneous coronary intervention OCT analysis modified the operator's strategy for management in 30% of cases. Treatment involved drug-eluting stent implantation in 61.6% and drug-eluting balloon angioplasty in 36.1% of cases with only 63.2% optimal results. The 1-year target vessel failure incidence was 11% (95% CI, 9%-13%). Residual postpercutaneous coronary intervention stent underexpansion was associated with significantly higher target vessel failure incidence (19% [95% CI, 14%-24%] versus 7% [95% CI, 5-9], P=0.01).
Conclusions: OCT identified neoatherosclerosis and neointimal hyperplasia in comparable proportions. Stent underexpansion was frequent and favored subsequent adverse clinical outcomes.
Keywords: in‐stent restenosis; optical coherence tomography; underexpansion.
Figures


References
-
- Yıldız A, Yıldız C, Oduncu V, Çimen AO. A comparison of drug‐eluting stent versus balloon angioplasty in patients with bare‐metal stent instent restenosis: 5 year outcomes. Int J Cardiovasc Acad. 2016;2:1–5. doi: 10.1016/j.ijcac.2016.02.003 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

