Piezo1-Mediated Mechanotransduction Contributes to Disturbed Flow-Induced Atherosclerotic Endothelial Inflammation
- PMID: 39450718
- PMCID: PMC11935715
- DOI: 10.1161/JAHA.123.035558
Piezo1-Mediated Mechanotransduction Contributes to Disturbed Flow-Induced Atherosclerotic Endothelial Inflammation
Abstract
Background: Disturbed flow generates oscillatory shear stress (OSS), which in turn leads to endothelial inflammation and atherosclerosis. Piezo1, a biomechanical force sensor, plays a crucial role in the cardiovascular system. However, the specific role of Piezo1 in atherosclerosis remains to be fully elucidated.
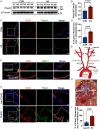
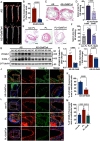
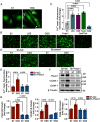
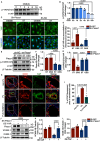
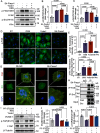
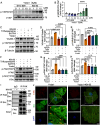
Methods and results: We detected the expression of Piezo1 in atherosclerotic mice and endothelial cells from regions with disturbed blood flow. The pharmacological inhibitor Piezo1 inhibitor (GsMTx4) was used to evaluate the impact of Piezo1 on plaque progression and endothelial inflammation. We examined Piezo1's direct response to OSS in vitro and its effects on endothelial inflammation. Furthermore, mechanistic studies were conducted to explore the potential molecular cascade through which Piezo1 mediates endothelial inflammation in response to OSS. Our findings revealed the upregulation of Piezo1 in apoE-/- (apolipoprotein E) atherosclerotic mice, which is associated with disturbed flow. Treatment with GsMTx4 not only delayed plaque progression but also mitigated endothelial inflammation in both chronic and disturbed flow-induced atherosclerosis. Piezo1 was shown to facilitate calcium ions (Ca2+) influx in response to OSS, thereby activating endothelial inflammation. This inflammatory response was attenuated in the absence of Piezo1. Additionally, we identified that under OSS, Piezo1 activates the Ca2+/CaM/CaMKII (calmodulin/calmodulin-dependent protein kinases Ⅱ) pathways, which subsequently stimulate downstream kinases FAK (focal adhesion kinase) and Src. This leads to the activation of the OSS-sensitive YAP (yes-associated protein), ultimately triggering endothelial inflammation.
Conclusions: Our study highlights the key role of Piezo1 in atherosclerotic endothelial inflammation, proposing the Piezo1-Ca2+/CaM/CaMKII-FAK/Src-YAP axis as a previously unknown endothelial mechanotransduction pathway. Piezo1 is expected to become a potential therapeutic target for atherosclerosis and cardiovascular diseases.
Keywords: Piezo1; atherosclerosis; endothelial cells; inflammation; shear stress.
Figures

References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

