The dual Ras-association domains of Drosophila Canoe have differential roles in linking cell junctions to the cytoskeleton during morphogenesis
- PMID: 39450902
- PMCID: PMC11698047
- DOI: 10.1242/jcs.263546
The dual Ras-association domains of Drosophila Canoe have differential roles in linking cell junctions to the cytoskeleton during morphogenesis
Abstract
During development cells must change shape and move without disrupting dynamic tissue architecture. This requires robust linkage of cell-cell adherens junctions to the force-generating actomyosin cytoskeleton. Drosophila Canoe and mammalian afadin play key roles in the regulation of such linkages. One central task for the field is defining mechanisms by which upstream inputs from Ras-family GTPases regulate Canoe and afadin. These proteins are unusual in sharing two tandem Ras-association (RA) domains - RA1 and RA2 - which when deleted virtually eliminate Canoe function. Work in vitro has suggested that RA1 and RA2 differ in GTPase affinity, but their individual functions in vivo remain unknown. Combining bioinformatic and biochemical approaches, we find that both RA1 and RA2 bind to active Rap1 with similar affinities, and that their conserved N-terminal extensions enhance binding. We created Drosophila canoe mutants to test RA1 and RA2 function in vivo. Despite their similar affinities for Rap1, RA1 and RA2 play strikingly different roles. Deleting RA1 virtually eliminates Canoe function, whereas mutants lacking RA2 are viable and fertile but have defects in junctional reinforcement in embryos and during pupal eye development. These data significantly expand our understanding of the regulation of adherens junction-cytoskeletal linkages.
Keywords: Adherens junction; Afadin; Canoe; GTPases; Morphogenesis; Rap1.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


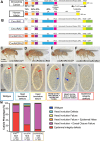

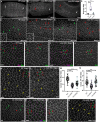
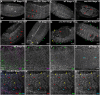
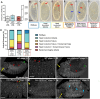

References
-
- Blackie, L., Tozluoglu, M., Trylinski, M., Walther, R. F., Schweisguth, F., Mao, Y. and Pichaud, F. (2021). A combination of Notch signaling, preferential adhesion and endocytosis induces a slow mode of cell intercalation in the Drosophila retina. Development 148, dev197301. 10.1242/dev.197301 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

