RNA mis-splicing in children with congenital myotonic dystrophy is associated with physical function
- PMID: 39450929
- PMCID: PMC11651218
- DOI: 10.1002/acn3.52224
RNA mis-splicing in children with congenital myotonic dystrophy is associated with physical function
Abstract
Objectives: Dysregulated RNA alternative splicing is the hallmark of myotonic dystrophy type 1 (DM1). However, the association between RNA mis-splicing and physical function in children with the most severe form of disease, congenital myotonic dystrophy (CDM), is unknown.
Methods: Eighty-two participants (42 adults with DM1 and 40 children with CDM) with muscle biopsies and measures of myotonia, motor function, and strength were combined from five observational studies. Data were normalized and correlated with an aggregate measure of alternative splicing dysregulation, [MBNL]inferred, in skeletal muscle biopsies. Multiple linear regression analysis was performed to predict [MBNL]inferred using clinical outcome measures alone. Similar analyses were performed to predict 12-month physical function using baseline metrics.
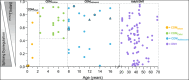
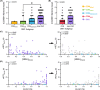
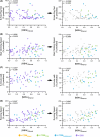
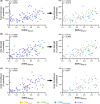
Results: Myotonia (measured via vHOT) was significantly correlated with RNA mis-splicing in our cross-sectional population of all DM1 individuals; CDM participants alone displayed no myotonia despite a similar range of RNA mis-splicing. Measures of motor performance and muscle strength were significantly associated with [MBNL]inferred in our cohort of all DM1 individuals and when assessing children with CDM independently. Multiple linear regression analyses yielded two models capable of predicting [MBNL]inferred from select clinical outcome assessments alone in all subjects (adjusted R2 = 0.6723) or exclusively in children with CDM (adjusted R2 = 0.5875).
Interpretation: Our findings establish significant correlations between skeletal muscle performance and a composite measure of alternative splicing dysregulation, [MBNL]inferred, in DM1. The strength of these correlations and the development of predictive models will assist in designing efficacious clinical trials for individuals with DM1, particularly CDM.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Julia M. Hartman, Marina Provenzano, Kameron Bates, Kobe Ikegami, Amanda Butler, Aileen S. Jones, Kiera N. Berggren, Marnee J. McKay, Jennifer N. Baldwin, and Kayla M.D. Cornett – None. Jeanne Dekdebrun – Consultation for Avidity Biosciences, Dyne Therapeutics, Vertex, Lupin, Arthex, PepGen and Trins. Joshua Burns – Research Support from the University of Sydney, Sydney Children's Hospitals Network, Australian Government (NHMRC#2015970, MRFF#1152226), United States Government (NIH NINDS#1U01NS109403, NIH NCATS/NINDS# U54NS065712), Muscular Dystrophy Association, American Orthotic and Prosthetic Association, Charcot Marie Tooth Association and Charcot Marie Tooth Australia. Scientific Advisory Board fees from Faculty of Medicine Siriraj Hospital Mahidol University Thailand; Department of Rehabilitation Sciences, The Hong Kong Polytechnic University; Hereditary Neuropathy Foundation. Consulted for DTx Pharma, Applied Therapeutics, Pharnext. Michael Kiefer – Has provided consultation for Aspa therapeutics. Nicholas E. Johnson – He has received grant funding from NINDS (R01NS104010, U01NS124974), NCATS (R21TR003184), CDC (U01DD001242) and the FDA (7R01FD006071). He receives royalties from the CCMDHI and the CMTHI. He receives research funds from Novartis, Takeda, PepGen, Sanofi Genzyme, Dyne, Vertex Pharmaceuticals, Fulcrum Therapeutics, AskBio, ML Bio, and Sarepta. He has provided consultation for Arthex, Angle Therapeutics, Juvena, Rgenta, PepGen, AMO Pharma, Takeda, Design, Dyne, AskBio, Avidity, and Vertex Pharmaceuticals. Melissa A. Hale – She has provided consultation for Juvena and Arrakis Therapeutics.
Figures


Update of
-
RNA mis-splicing in children with myotonic dystrophy is associated with physical function.bioRxiv [Preprint]. 2024 Jul 3:2024.07.03.600889. doi: 10.1101/2024.07.03.600889. bioRxiv. 2024. Update in: Ann Clin Transl Neurol. 2024 Dec;11(12):3175-3191. doi: 10.1002/acn3.52224. PMID: 39109179 Free PMC article. Updated. Preprint.
References
-
- Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255(5049):1253‐1255. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255(5049):1256‐1258. - PubMed
-
- Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68(4):799‐808. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

