Objective detection of wheeze at home by parents through a digital device: usage patterns and relationship with SABA administration
- PMID: 39451025
- PMCID: PMC11715141
- DOI: 10.1002/ppul.27295
Objective detection of wheeze at home by parents through a digital device: usage patterns and relationship with SABA administration
Abstract
Introduction: Wheezing is an important indicator of exacerbated respiratory symptoms in early childhood and must be monitored to regulate pharmacological therapy. However, parents' subjective perception of wheezing in their children is not always precise. We investigated the objective identification of children's wheezing by parents using a digital wheeze detector (WheezeScanTM, OMRON Healthcare Co. Ltd), its longitudinal usage patterns, and its relationship with SABA administration.
Methods: We conducted a secondary nested analysis of data from the intervention arm of a multi-center randomized controlled trial completed in 2021-2022 in Berlin (Germany), London (United Kingdom), and Istanbul (Turkey). Children aged 4 to 84 months with doctor's diagnosed wheezing (GINA step 1 or 2) were included. Using an electronic diary (Wheeze-MonitorTM, TPS), parents monitored and recorded for 120 days at home the presence or absence of their child's wheezing, detected both, with WheezeScanTM ("objective" wheezing), and subjective ("perceived" wheezing). Parents also recorded the child's symptoms, medication intake, and family quality of life. Questionnaires regarding symptom control, quality of life, and parental self-efficacy were answered at baseline and after 90 and 120 days.
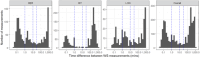
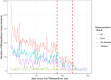
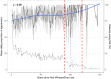
Results: Eighty-one/87 families completed the intervention arm of the study. WheezeScanTM was on average used 0.7 (SD 0.6) times a day, with each patient reporting a positive, negative, or "error" outcome on average in 57%, 39%, and 5% of measurements, respectively. The use of WheezeScanTM declined slightly during the first 90 days of monitoring and steeply thereafter. Repeated usage of WheezeScanTM in the same day was more frequent after a "wheeze" (HR 1.5, 95% CI 1.37-1.65, p < 0.001) and an "error" (HR 2.01, 95% CI 1.70-2.38, p < 0.001) result, compared to a "no wheeze" outcome. The average per-patient daily agreement between "objective" and "perceived" wheezing/non-wheezing was 75% at the start of the monitoring period and only weakly persisted as time passed (Spearman's rho=0.09). The frequency of short-acting beta-2-agonists (SABA) administration was lower in days with closely interspaced consecutive device uses during which the patient's status was perceived as "never wheeze" (32/455, 7%) than in those perceived as "persistent wheeze" (53/119, 44%; OR 36.6, 95% CI [14.3, 94.1]).
Conclusion: Daily use of a digital WheezeScanTM at home allows parents to detect their child's unperceived wheezing and discloses to caregivers the longitudinal patterns of a child's wheezing disorder. Digital monitoring of wheezing also highlights poor adherence to guidelines in SABA administration for wheezing children, with under-treatment being much more frequent than over-treatment. This pioneering study opens new perspectives for further investigation of digital wheeze detectors in the early diagnosis and proper self-management of wheezing disorders in childhood.
Keywords: childhood; digital health; pre‐school wheezing; self‐management; wheeze detector.
© 2024 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
Tina‐Maria Weichert, Siri Roßberg, Claude Grenzbach, Ellen Dellbrügger, Bulent Karadag, and Abigail Whitehouse have received support for the work on this study via an unrestricted scientific grant. Ulrike Grittner has received support for her work on this study as a biostatistician from OMRON Healthcare Co., Ltd via an unrestricted scientific grant. Stephanie Dramburg and Jonathan Grigg received speaker fees from OMRON Healthcare. Paolo Maria Matricardi has received grant funding and provision of study material from OMRON Health Care for the present study as well as consulting fees from OMRON Healthcare not related to the present study. Camilo Jose Hernandez Toro, Yen Hoang Do, Wim van Aalderen and Eric Haarman have nothing to disclose.
Figures


References
-
- Doss AMA, Stokes JR. Viral infections and wheezing in preschool children. Immunol Allergy Clin North Am. 2022;42(4):727‐741. - PubMed
-
- Brand PLP, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence‐based approach. Eur Respir J. 2008;32(4):1096‐1110. - PubMed
-
- Brand PLP, Caudri D, Eber E, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J. 2014;43(4):1172‐1177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

