Long-Term Human Immune Reconstitution, T-Cell Development, and Immune Reactivity in Mice Lacking the Murine Major Histocompatibility Complex: Validation with Cellular and Gene Expression Profiles
- PMID: 39451205
- PMCID: PMC11506606
- DOI: 10.3390/cells13201686
Long-Term Human Immune Reconstitution, T-Cell Development, and Immune Reactivity in Mice Lacking the Murine Major Histocompatibility Complex: Validation with Cellular and Gene Expression Profiles
Abstract
Background: Humanized mice transplanted with CD34+ hematopoietic cells (HPCs) are broadly used to study human immune responses and infections in vivo and for testing therapies pre-clinically. However, until now, it was not clear whether interactions between the mouse major histocompatibility complexes (MHCs) and/or the human leukocyte antigens (HLAs) were necessary for human T-cell development and immune reactivity.
Methods: We evaluated the long-term (20-week) human hematopoiesis and human T-cell development in NOD Scid Gamma (NSG) mice lacking the expression of MHC class I and II (NSG-DKO). Triplicate experiments were performed with HPCs obtained from three donors, and humanization was confirmed in the reference strain NOD Rag Gamma (NRG). Further, we tested whether humanized NSG-DKO mice would respond to a lentiviral vector (LV) systemic delivery of HLA-A*02:01, HLA-DRB1*04:01, human GM-CSF/IFN-α, and the human cytomegalovirus gB antigen.
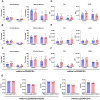
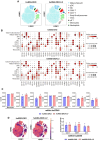
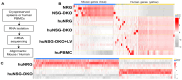
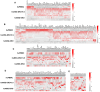
Results: Human immune reconstitution was detectable in peripheral blood from 8 to 20 weeks after the transplantation of NSG-DKO. Human single positive CD4+ and CD8+ T-cells were detectable in lymphatic tissues (thymus, bone marrow, and spleen). LV delivery harnessed the detection of lymphocyte subsets in bone marrow (αβ and γδ T-cells and NK cells) and the expression of HLA-DR. Furthermore, RNA sequencing showed that LV delivery increased the expression of different human reactome pathways, such as defense responses to other organisms and viruses.
Conclusions: Human T-cell development and reactivity are independent of the expression of murine MHCs in humanized mice. Therefore, humanized NSG-DKO is a promising new model for studying human immune responses, as it abrogates the xenograft mouse MHC interference.
Keywords: HLA; IFN; MHC; NSG; T-cell; humanized mice; immunization; lentivirus; stem cell transplantation.
Conflict of interest statement
R.S. received honoraria for participating and organizing conferences with The Jackson Laboratory, a not-for-profit organization commercializing the mouse strains used in these studies. L.S. and B.S. are employees of The Jackson Laboratory. The other authors declare no conflicts of interest.
Figures





References
-
- Götze D., Burger R. The Major Histocompatibility Complex. In: Bier O.G., Dias Da Silva W., Götze D., Mota I., editors. Fundamentals of Immunology. Springer; Berlin/Heidelberg, Germany: 1986. pp. 139–178.
-
- Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M. Development of Functional Human Blood and Immune Systems in NOD/SCID/IL2 Receptor γ Chain(Null) Mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

