Accelerated Aging Effects Observed In Vitro after an Exposure to Gamma-Rays Delivered at Very Low and Continuous Dose-Rate Equivalent to 1-5 Weeks in International Space Station
- PMID: 39451221
- PMCID: PMC11506070
- DOI: 10.3390/cells13201703
Accelerated Aging Effects Observed In Vitro after an Exposure to Gamma-Rays Delivered at Very Low and Continuous Dose-Rate Equivalent to 1-5 Weeks in International Space Station
Abstract
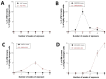
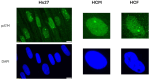
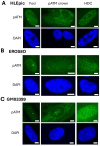
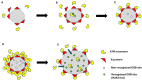
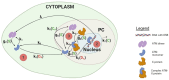
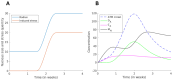
Radiation impacting astronauts in their spacecraft come from a "bath" of high-energy rays (0.1-0.5 mGy per mission day) that reaches deep tissues like the heart and bones and a "stochastic rain" of low-energy particles from the shielding and impacting surface tissues like skin and lenses. However, these two components cannot be reproduced on Earth together. The MarsSimulator facility (Toulouse University, France) emits, thanks to a bag containing thorium salts, a continuous exposure of 120 mSv/y, corresponding to that prevailing in the International Space Station (ISS). By using immunofluorescence, we assessed DNA double-strand breaks (DSB) induced by 1-5 weeks exposure in ISS of human tissues evoked above, identified at risk for space exploration. All the tissues tested elicited DSBs that accumulated proportionally to the dose at a tissue-dependent rate (about 40 DSB/Gy for skin, 3 times more for lens). For the lens, bones, and radiosensitive skin cells tested, perinuclear localization of phosphorylated forms of ataxia telangiectasia mutated protein (pATM) was observed during the 1st to 3rd week of exposure. Since pATM crowns were shown to reflect accelerated aging, these findings suggest that a low dose rate of 120 mSv/y may accelerate the senescence process of the tested tissues. A mathematical model of pATM crown formation and disappearance has been proposed. Further investigations are needed to document these results in order to better evaluate the risks related to space exploration.
Keywords: ATM protein; DNA double-strand breaks; accelerated aging; astronauts; risks; space radiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Cucinotta F.A., Kim M.-H.Y., Ren L. Managing Luna and Mars Mission Radiation Risks. Part I; Cancer Risks, Uncertainties and Shielding Effectiveness. NASA; Hanover, MD, USA: 2005. NASA Report. TP-2005-213164.
-
- Cucinotta F.A., Schimmerling W., Wilson J.W., Peterson L.E., Badhmar G.D., Saganti P.B., Dicello J.F. Space Radiation Cancer Risk Projections for Exploration Missions: Uncertainty Reduction and Mitigation. NASA; Hanover, MD, USA: 2011. NASA Report. JSC-29295.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

