Difenoconazole Induced Damage of Bovine Mammary Epithelial Cells via ER Stress and Inflammatory Response
- PMID: 39451231
- PMCID: PMC11506304
- DOI: 10.3390/cells13201715
Difenoconazole Induced Damage of Bovine Mammary Epithelial Cells via ER Stress and Inflammatory Response
Abstract
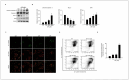
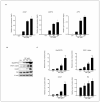
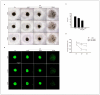
Difenoconazole (DIF) is a fungicide used to control various fungi. It is absorbed on the surface of different plants and contributes significantly to increased crop production. However, DIF is reported to exhibit toxicity to fungi and to aquatic plants, fish, and mammals, including humans, causing adverse effects. However, research on the impact of DIF on the mammary epithelial cells of herbivorous bovines is limited. DIF-induced damage and accumulation in the mammary glands can have direct and indirect effects on humans. Therefore, we investigated the effects and mechanisms of DIF toxicity in MAC-T cells. The current study revealed that DIF reduces cell viability and proliferation while triggering apoptotic cell death through the upregulation of pro-apoptotic proteins, including cleaved caspase 3 and Bcl-2-associated X protein (BAX), and the downregulation of leukemia type 2 (BCL-2). DIF also induced endoplasmic reticulum (ER) stress by increasing the expression of genes or proteins of Bip/GRP78, protein disulfide isomerase (PDI), activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), and endoplasmic reticulum oxidoreductase 1 Alpha (ERO1-Lα). We demonstrated that DIF induces mitochondria-mediated apoptosis in MAC-T cells by activating ER stress pathways. This cellular damage resulted in a significant increase in the expression of inflammatory response genes and proteins, including cyclooxygenase 2 (COX2), transforming growth factor beta 3 (TGFB3), CCAAT enhancer binding protein delta (CEBPD), and iNOS, in DIF-treated groups. In addition, spheroid formation by MAC-T cells was suppressed by DIF treatment. Our findings suggest that DIF exposure in dairy cows may harm mammary gland function and health and may indirectly affect human consumption of milk.
Keywords: ER stress; bovine mammary gland; difenoconazole; inflammation.
Conflict of interest statement
The authors declare that they do not have any conflicts of interests.
Figures


References
-
- Schäfer R.B., Pettigrove V., Rose G., Allinson G., Wightwick A., von der Ohe P.C., Shimeta J., Kühne R., Kefford B.J. Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ. Sci. Technol. 2011;45:1665–1672. doi: 10.1021/es103227q. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

