The Neural Correlations of Olfactory Associative Reward Memories in Drosophila
- PMID: 39451234
- PMCID: PMC11506542
- DOI: 10.3390/cells13201716
The Neural Correlations of Olfactory Associative Reward Memories in Drosophila
Abstract
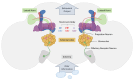
Advancing treatment to resolve human cognitive disorders requires a comprehensive understanding of the molecular signaling pathways underlying learning and memory. While most organ systems evolved to maintain homeostasis, the brain developed the capacity to perceive and adapt to environmental stimuli through the continuous modification of interactions within a gene network functioning within a broader neural network. This distinctive characteristic enables significant neural plasticity, but complicates experimental investigations. A thorough examination of the mechanisms underlying behavioral plasticity must integrate multiple levels of biological organization, encompassing genetic pathways within individual neurons, interactions among neural networks providing feedback on gene expression, and observable phenotypic behaviors. Model organisms, such as Drosophila melanogaster, which possess more simple and manipulable nervous systems and genomes than mammals, facilitate such investigations. The evolutionary conservation of behavioral phenotypes and the associated genetics and neural systems indicates that insights gained from flies are pertinent to understanding human cognition. Rather than providing a comprehensive review of the entire field of Drosophila memory research, we focus on olfactory associative reward memories and their related neural circuitry in fly brains, with the objective of elucidating the underlying neural mechanisms, thereby advancing our understanding of brain mechanisms linked to cognitive systems.
Keywords: Drosophila melanogaster; brain; neural circuits; reward memories.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

