Multispectral Imaging of Collagen, NAD(P)H and Flavin Autofluorescence in Mesenchymal Stem Cells Undergoing Trilineage Differentiation
- PMID: 39451249
- PMCID: PMC11505937
- DOI: 10.3390/cells13201731
Multispectral Imaging of Collagen, NAD(P)H and Flavin Autofluorescence in Mesenchymal Stem Cells Undergoing Trilineage Differentiation
Abstract
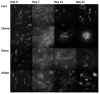
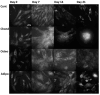
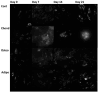
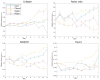
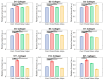
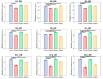
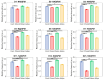
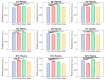
Understanding the molecular mechanisms of differentiation is important for regenerative medicine and developmental biology. This study aims to characterise the role of the glycolysis/oxidative phosphorylation balance as a driver of mesenchymal stem cell (MSC) differentiation. Cells were maintained in normal conditions or stimulated towards the MSC trilineage cell types over 21 days. Multispectral imaging of cell autofluorescence was applied as a non-invasive methodology to continuously image cultures in situ. Spectral signals for collagen, NAD(P)H, and flavins were unmixed. MSCs cultured under chondrogenic conditions exhibited increased collagen levels relative to controls. Following osteogenic induction, MSCs showed increased collagen levels relative to controls during the earlier stages of culture; however, control cells increased their collagen levels as they became confluent. MSCs cultured under adipogenic conditions exhibited lower levels of collagen than controls. The redox ratio (RR; NAD(P)H/flavins) immediately decreased during chondrogenesis, with this early effect persisting throughout the culture compared to control cells, which appeared to increase their RR, similar to osteogenesis. Adipogenesis resulted in a small increase in RR on day 2 relative to control cells, followed by a persistent decrease. Chondrogenic and adipogenic differentiation favoured oxidative phosphorylation, whereas osteogenesis and MSC overgrowth resulted in a glycolytic metabolism. Following consideration of these findings, as well as the diverse reports in the literature, it is concluded that neither enhanced oxidative phosphorylation nor glycolysis are fundamental to the canonical modes of differentiation, and researchers should avoid interpreting shifts as indicating differentiation.
Keywords: autofluorescence; differentiation; mesenchymal; spectroscopy; stem cells.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18:759–765. doi: 10.1038/nm.2736. - DOI - PMC - PubMed
-
- Meleshina A.V., Dudenkova V.V., Bystrova A.S., Kuznetsova D.S., Shirmanova M.V., Zagaynova E.V. Two-photon FLIM of NAD(P)H and FAD in mesenchymal stem cells undergoing either osteogenic or chondrogenic differentiation. Stem Cell Res. Ther. 2017;8:15. doi: 10.1186/s13287-017-0484-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

