DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival
- PMID: 39451259
- PMCID: PMC11506473
- DOI: 10.3390/cells13201742
DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival
Abstract
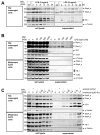
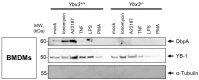
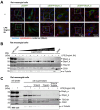
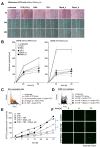
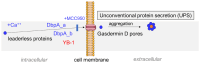
DNA-binding protein A (DbpA) belongs to the Y-box family of cold shock domain (CSD) proteins that bind RNA/DNA and exert intracellular functions in cell stress, proliferation, and differentiation. Given the pattern of DbpA staining in inflammatory glomerular diseases, without adherence to cell boundaries, we hypothesized extracellular protein occurrence and specific functions. Lipopolysaccharide and ionomycin induce DbpA expression and secretion from melanoma and mesangial cells. Unlike its homologue Y-box-binding protein 1 (YB-1), DbpA secretion requires inflammasome activation, as secretion is blocked upon the addition of a NOD-like receptor protein-3 (NLRP3) inhibitor. The addition of recombinant DbpA enhances melanoma cell proliferation, migration, and competes with tumor necrosis factor (TNF) binding to its receptor (TNFR1). In TNF-induced cell death assays, rDbpA initially blocks TNF-induced apoptosis, whereas at later time points (>24 h), cells are more prone to die. Given that CSD proteins YB-1 and DbpA fulfill the criteria of alarmins, we propose that their release signals an inherent danger to the host. Some data hint at an extracellular complex formation at a ratio of 10:1 (DbpA:YB-1) of both proteins.
Keywords: cold shock domain proteins; danger signal; inflammation; innate immunity; protein secretion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- Project-ID 97850925 - SFB 854: PRM (Project A01)/Deutsche Forschungsgemeinschaft
- Project-ID 361210922/RTG 2408 project 8-1, MD10/Deutsche Forschungsgemeinschaft
- ME-1365/7-2 (Project-ID 17844716)/Deutsche Forschungsgemeinschaft
- ME-1365/9-3 (Project-ID 260632335)/Deutsche Forschungsgemeinschaft
- BR3481/7-1 (Project-ID 517830864)/Deutsche Forschungsgemeinschaft
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

