Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis
- PMID: 39451279
- PMCID: PMC11507190
- DOI: 10.3390/gels10100626
Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis
Abstract
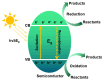
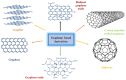
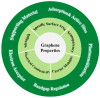
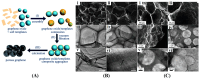
Converting solar energy into fuels/chemicals through photochemical approaches holds significant promise for addressing global energy demands. Currently, semiconductor photocatalysis combined with redox techniques has been intensively researched in pollutant degradation and secondary energy generation owing to its dual advantages of oxidizability and reducibility; however, challenges remain, particularly with improving conversion efficiency. Since graphene's initial introduction in 2004, three-dimensional (3D) graphene-based photocatalysts have garnered considerable attention due to their exceptional properties, such as their large specific surface area, abundant pore structure, diverse surface chemistry, adjustable band gap, and high electrical conductivity. Herein, this review provides an in-depth analysis of the commonly used photocatalysts based on 3D graphene, outlining their construction strategies and recent applications in photocatalytic degradation of organic pollutants, H2 evolution, and CO2 reduction. Additionally, the paper explores the multifaceted roles that 3D graphene plays in enhancing photocatalytic performance. By offering a comprehensive overview, we hope to highlight the potential of 3D graphene as an environmentally beneficial material and to inspire the development of more efficient, versatile graphene-based aerogel photocatalysts for future applications.
Keywords: aerogel; application; graphene; photocatalyst.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





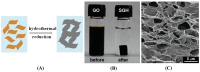



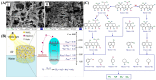
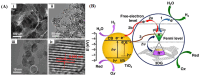

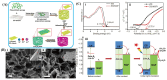
References
-
- Mei W., Lu H., Dong R., Tang S., Xu J. Ce-Doped Brookite TiO2 Quasi-Nanocubes for Efficient Visible Light Catalytic Degradation of Tetracycline. ACS Appl. Nano Mater. 2024;7:1825–1834. doi: 10.1021/acsanm.3c05072. - DOI
-
- Wang Y., Jia K., Pan Q., Xu Y., Liu Q., Cui G., Guo X., Sun X. Boron-Doped TiO2 for Efficient Electrocatalytic N2 Fixation to NH3 at Ambient Conditions. ACS Sustain. Chem. Eng. 2018;7:117–122. doi: 10.1021/acssuschemeng.8b05332. - DOI
-
- Basyach P., Prajapati P.K., Rohman S.S., Sonowal K., Kalita L., Malik A., Guha A.K., Jain S.L., Saikia L. Visible Light-Active Ternary Heterojunction Photocatalyst for Efficient CO2 Reduction with Simultaneous Amine Oxidation and Sustainable H2O2 Production. ACS Appl. Mater. Interfaces. 2023;15:914–931. doi: 10.1021/acsami.2c16549. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

