Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema
- PMID: 39451340
- PMCID: PMC11505434
- DOI: 10.3390/bioengineering11100964
Dynamics of Treatment Response to Faricimab for Diabetic Macular Edema
Abstract
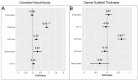
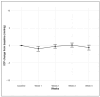
This study analyzes the dynamics of short-term treatment response to the first intravitreal faricimab injection in eyes with diabetic macular edema (DME). This retrospective, single-center, clinical trial was conducted at the Department of Ophthalmology, University Hospital Zurich. Patients with treatment-naïve and pretreated DME were included. Patient chart data and imaging were analyzed. Safety and efficacy (corrected visual acuity (CVA), central subfield thickness (CST), and signs of intraocular inflammation (IOI)) of the first faricimab intravitreal therapy (IVT) were evaluated weekly until 4 weeks after injection. Forty-three eyes (81% pretreated) of 31 patients were included. Four weeks after the first faricimab IVT, CVA remained stable and median CST (µm) decreased significantly (p < 0.001) from 325.0 (293.5-399.0) at baseline to 304.0 (286.5-358.0). CVA at week 4 was only associated with baseline CVA (p < 0.001). CST was the only predictive variable (p = 0.002) between baseline and week 4 CST. Weekly safety assessments did not show any sign of clinically significant IOI. This study suggests faricimab is an effective treatment for (pretreated) DME, showing structural benefit 1 month following the first injection without short-term safety signals.
Keywords: DME; IOI; IVI; IVT; ang-2; anti-VEGF; diabetic macular edema; faricimab; intraocular inflammation; intravitreal therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wells J.A., Glassman A.R., Ayala A.R., Jampol L.M., Bressler N.M., Bressler S.B., Brucker A.J., Ferris F.L., Hampton G.R., Jhaveri C., et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351–1359. doi: 10.1016/j.ophtha.2016.02.022. - DOI - PMC - PubMed
-
- Diabetic Retinopathy Clinical Research Network. Wells J.A., Glassman A.R., Ayala A.R., Jampol L.M., Aiello L.P., Antoszyk A.N., Arnold-Bush B., Baker C.W., Bressler N.M., et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015;372:1193–1203. doi: 10.1016/j.ophtha.2016.02.022. - DOI - PMC - PubMed
-
- Moja L., Lucenteforte E., Kwag K.H., Bertele V., Campomori A., Chakravarthy U., D’Amico R., Dickersin K., Kodjikian L., Lindsley K., et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014;9:CD011230. doi: 10.1002/14651858.CD011230.pub2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

