Interplay of Glucose Metabolism and Hippo Pathway in Chondrocytes: Pathophysiology and Therapeutic Targets
- PMID: 39451348
- PMCID: PMC11505586
- DOI: 10.3390/bioengineering11100972
Interplay of Glucose Metabolism and Hippo Pathway in Chondrocytes: Pathophysiology and Therapeutic Targets
Abstract
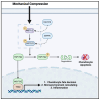
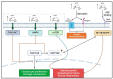
In this review, we explore the intricate relationship between glucose metabolism and mechanotransduction pathways, with a specific focus on the role of the Hippo signaling pathway in chondrocyte pathophysiology. Glucose metabolism is a vital element in maintaining proper chondrocyte function, but it has also been implicated in the pathogenesis of osteoarthritis (OA) via the induction of pro-inflammatory signaling pathways and the establishment of an intracellular environment conducive to OA. Alternatively, mechanotransduction pathways such as the Hippo pathway possess the capacity to respond to mechanical stimuli and have an integral role in maintaining chondrocyte homeostasis. However, these mechanotransduction pathways can be dysregulated and potentially contribute to the progression of OA. We discussed how alterations in glucose levels may modulate the Hippo pathway components via a variety of mechanisms. Characterizing the interaction between glucose metabolism and the Hippo pathway highlights the necessity of balancing both metabolic and mechanical signaling to maintain chondrocyte health and optimal functionality. Furthermore, this review demonstrates the scarcity of the literature on the relationship between glucose metabolism and mechanotransduction and provides a summary of current research dedicated to this specific area of study. Ultimately, increased research into this topic may elucidate novel mechanisms and relationships integrating mechanotransduction and glucose metabolism. Through this review we hope to inspire future research into this topic to develop innovative treatments for addressing the clinical challenges of OA.
Keywords: OA therapeutics; chondrocyte; glucose; mechanotransduction; metabolism; osteoarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

