Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli
- PMID: 39451351
- PMCID: PMC11504287
- DOI: 10.3390/bioengineering11100975
Preparation and Characterization of Hydroxylated Recombinant Collagen by Incorporating Proline and Hydroxyproline in Proline-Deficient Escherichia coli
Abstract
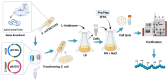
Collagen possesses distinctive chemical properties and biological functions due to its unique triple helix structure. However, recombinant collagen expressed in Escherichia coli without post-translational modifications such as hydroxylation lacks full function since hydroxylation is considered to be critical to the stability of the collagen triple-helix at body temperature. Here, a proline-deficient E. coli strain was constructed and employed to prepare hydroxylated recombinant collagens by incorporating proline (Pro) and hydroxyproline (Hyp) from the culture medium. By controlling the ratio of Pro to Hyp in the culture medium, collagen with different degrees of hydroxylation (0-88%) can be obtained. When the ratio of Pro and Hyp was adjusted to 12:8 mM, the proline hydroxylation rate of recombinant human collagen (rhCol, 55 kDa) ranged from 40-50%, which was also the degree of natural collagen. After proline hydroxylation, both the thermal stability and cell binding of rhCol were significantly enhanced. Notably, when the hydroxylation rate approached that of native human collagen (40-50%), the improvements were most pronounced. Moreover, the cell binding of rhCol with a hydroxylation rate of 43% increased by 29%, and the melting temperature (Tm) rose by 5 °C compared to the non-hydroxylated rhCol. The system achieved a yield of 1.186 g/L of rhCol by batch-fed in a 7 L fermenter. This innovative technology is expected to drive the development and application of collagen-related biomaterials with significant application value in the fields of tissue engineering, regenerative medicine, and biopharmaceuticals.
Keywords: co-expression; hydroxylation rate; incorporation; recombinant collagen; triple helix.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Recombinant expression of hydroxylated human collagen in Escherichia coli.Appl Microbiol Biotechnol. 2014 May;98(10):4445-55. doi: 10.1007/s00253-013-5447-z. Epub 2013 Dec 21. Appl Microbiol Biotechnol. 2014. PMID: 24362857
-
Glycosylation/Hydroxylation-induced stabilization of the collagen triple helix. 4-trans-hydroxyproline in the Xaa position can stabilize the triple helix.J Biol Chem. 2000 Aug 11;275(32):24466-9. doi: 10.1074/jbc.M003336200. J Biol Chem. 2000. PMID: 10827193
-
Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase.BMC Biotechnol. 2011 Jun 24;11:69. doi: 10.1186/1472-6750-11-69. BMC Biotechnol. 2011. PMID: 21702901 Free PMC article.
-
Structural aspects of hydroxyproline-containing proteins.J Biomol Struct Dyn. 1983 Dec;1(3):843-55. doi: 10.1080/07391102.1983.10507485. J Biomol Struct Dyn. 1983. PMID: 6401122 Review.
-
Bioengineered Collagens.Subcell Biochem. 2017;82:601-629. doi: 10.1007/978-3-319-49674-0_18. Subcell Biochem. 2017. PMID: 28101874 Review.
References
-
- Parenteau N. Skin: The first tissue-engineered products. Sci. Am. 1999;280:83–84. - PubMed
-
- Shepherd J.H., Howard D., Waller A.K., Foster H.R., Mueller A., Moreau T., Evans A.L., Arumugam M., Chalon G.B., Vriend E. Structurally Graduated Collagen Scaffolds Applied to the Ex Vivo Generation of Platelets from Human Pluripotent Stem Cell-Derived Megakaryocytes: Enhancing Production and Purity. Biomaterials. 2018;182:135–144. doi: 10.1016/j.biomaterials.2018.08.019. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

