Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway
- PMID: 39451570
- PMCID: PMC11505814
- DOI: 10.3390/cimb46100690
Homosalate and ERK Knockdown in the Modulation of Aurelia coerulea Metamorphosis by Regulating the PI3K Pathway and ERK Pathway
Abstract
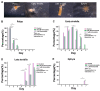
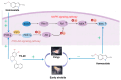
Metamorphosis control is pivotal in preventing the outbreak of jellyfish, and it is often studied using common model organisms. The widespread use of the ultraviolet blocking agent homosalate in cosmetics poses a threat to marine ecosystems. Although the impact of homosalate on marine organisms has been extensively examined, there is a notable absence of research on its effects on jellyfish metamorphosis and the underlying mechanisms, warranting further investigation. In this study, we first established a study model by using 5-methoxy-2-methylindole to induce Aurelia coerulea metamorphosis, and selected homosalate as a PI3K agonist and an ERK agonist, while we used YS-49 as a specific PI3K agonist, as well as ERK knockdown, to observe their effect on the metamorphosis of Aurelia coerulea. The results showed that an Aurelia coerulea metamorphosis model was established successfully, and the PI3K agonist homosalate, YS-49, and the knockdown of ERK molecules could significantly delay the metamorphosis development of Aurelia coerulea. We propose that activating PI3K/Akt and inhibiting the ERK pathway are involved in the delayed development of Aurelia coerulea, which provides a new strategy for the prevention and control of jellyfish blooms.
Keywords: Aurelia coerulea; ERK pathway; PI3K pathway; homosalate; metamorphosis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Copper-induced oxidative stress inhibits asexual reproduction of Aurelia coerulea polyps.Ecotoxicol Environ Saf. 2024 Oct 15;285:117112. doi: 10.1016/j.ecoenv.2024.117112. Epub 2024 Sep 26. Ecotoxicol Environ Saf. 2024. PMID: 39332202
-
The biogenic reefs formed by the alien polychaete Hydroides dianthus (Serpulidae, Annelida) favor the polyp stage of Aurelia coerulea (Cnidaria, Scyphozoa) in a coastal artificial lake.Mar Pollut Bull. 2018 Apr;129(1):86-91. doi: 10.1016/j.marpolbul.2018.02.016. Epub 2018 Feb 20. Mar Pollut Bull. 2018. PMID: 29680572
-
Combined effects of ocean acidification and temperature on planula larvae of the moon jellyfish Aurelia coerulea.Mar Environ Res. 2018 Aug;139:144-150. doi: 10.1016/j.marenvres.2018.05.015. Epub 2018 May 16. Mar Environ Res. 2018. PMID: 29789135
-
[Research advances in the effects of environmental factors on the growth and development of Aurelia spp].Ying Yong Sheng Tai Xue Bao. 2012 Nov;23(11):3207-17. Ying Yong Sheng Tai Xue Bao. 2012. PMID: 23431810 Review. Chinese.
-
Jellyfish blooms in China: Dominant species, causes and consequences.Mar Pollut Bull. 2010 Jul;60(7):954-63. doi: 10.1016/j.marpolbul.2010.04.022. Epub 2010 May 31. Mar Pollut Bull. 2010. PMID: 20553695 Review.
References
-
- Purcell J.E., Uye S.I., Lo W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007;350:153–174. doi: 10.3354/meps07093. - DOI
-
- Brotz L., Cheung W.L., Kleisner K., Pakhomov E., Pauly D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia. 2012;690:3–20. doi: 10.1007/s10750-012-1039-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

