Vascular Perspectives of the Midfacial Superficial Musculoaponeurotic System
- PMID: 39451617
- PMCID: PMC11507235
- DOI: 10.3390/diagnostics14202294
Vascular Perspectives of the Midfacial Superficial Musculoaponeurotic System
Abstract
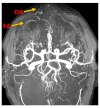
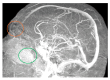
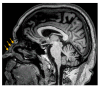
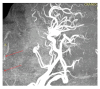
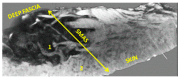
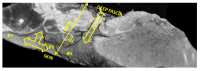
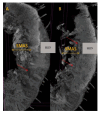
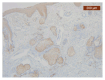
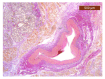
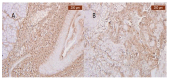
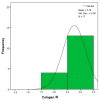
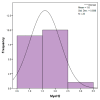
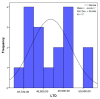
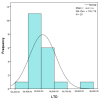
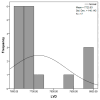
Objectives: Presently, data on the vascularization of the superficial musculoaponeurotic system of the face (SMAS) are lacking. Thus, the present study aimed to provide new conclusive data about the topography, density, and relationship of the SMAS blood vessels with other components, namely, the fibrous connective tissue and muscles. Methods: The study included a control lot of 42 cases from the archive of the radiology department. In this group, nuclear magnetic resonance angiography (MRA) was performed in order to identify the main sources of vascular supply. In the second group, tissue samples were collected from the midfacial region of 45 patients from the Oro-Maxillo-Facial and Plastic and Reconstructive Surgery clinics of 'St. Spiridon' County Clinical Emergency Hospital, Iasi. These patients received surgery for excision of tumoral formations that did not involve SMAS components. These samples underwent micro-CT analysis, hematoxylin and eosin (HE) staining, as well as immunohistochemical (IHC) staining for collagen type III, muscle tissue, and the vascular endothelium. Results: We discovered the particular way in which the SMAS components interrelate with vascularization and the regional differences between them. We have discovered a new vascular network specific to the SMAS, highlighted by both the micro-CT technique and microscopy on slides with special IHC staining. Significant differences were observed in the topographic arrangement, density, and relationships of the microscopic vasculature across midfacial regions. IHC staining provided morphological and functional information about the structure and vascularization of SMAS. Conclusions: The MRA technique could not detect the structural blood vessels of the SMAS and other methods for their in vivo visualization must be sought. The blood vessels of the SMAS mainly follow the topography of the muscle fibers. From the SMAS layer where they are found, the distribution branches reach the stroma of the region and the hypoderm. Our data can contribute to the development of surgical techniques tailored to each individual patient, as well as the enhancement of methods for stimulating cutaneous angiogenesis, improving scarring in this region, and advancing biotissue engineering techniques.
Keywords: MRA; SMAS; collagen; endothelium; vascular microperfusion of face.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
















Similar articles
-
Morphofunctional and histological patterns of blood vessels in the superficial cervicofacial musculoaponeurotic system in midlateral face regions.Ann Anat. 2024 Apr;253:152221. doi: 10.1016/j.aanat.2024.152221. Epub 2024 Feb 1. Ann Anat. 2024. PMID: 38309593
-
Immunohistochemical evidence of striated muscle cells within midfacial superficial musculoaponeurotic system.Ann Anat. 2021 Mar;234:151647. doi: 10.1016/j.aanat.2020.151647. Epub 2020 Nov 20. Ann Anat. 2021. PMID: 33221387
-
Aging of Superficial Musculoaponeurotic System of the Face-Novel Biomarkers and Micro-CT Relevance of Facial Anti-Gravity Support.Diagnostics (Basel). 2024 May 29;14(11):1126. doi: 10.3390/diagnostics14111126. Diagnostics (Basel). 2024. PMID: 38893653 Free PMC article.
-
Anatomy of the SMAS revisited.Aesthetic Plast Surg. 2003 Jul-Aug;27(4):258-64. doi: 10.1007/s00266-003-3065-3. Aesthetic Plast Surg. 2003. PMID: 15058546 Review.
-
Superficial Fascia in the Cheek and the Superficial Musculoaponeurotic System.J Craniofac Surg. 2018 Jul;29(5):1378-1382. doi: 10.1097/SCS.0000000000004585. J Craniofac Surg. 2018. PMID: 29621090 Review.
References
LinkOut - more resources
Full Text Sources

