Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection
- PMID: 39451693
- PMCID: PMC11506539
- DOI: 10.3390/bios14100480
Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection
Abstract
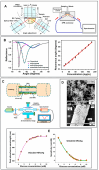
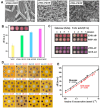
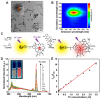
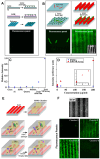
Significant research accomplishments have been made so far for the development and application of ZnO nanomaterials in enhanced optical biodetection. The unparalleled optical properties of ZnO nanomaterials and their reduced dimensionality have been successfully exploited to push the limits of conventional optical biosensors and optical biodetection platforms for a wide range of bioanalytes. ZnO nanomaterial-enabled advancements in optical biosensors have been demonstrated to improve key sensor performance characteristics such as the limit of detection and dynamic range. In addition, all nanomaterial forms of ZnO, ranging from 0-dimensional (0D) and 1D to 2D nanostructures, have been proven to be useful, ensuring their versatile fabrication into functional biosensors. The employment of ZnO as an essential biosensing element has been assessed not only for ensembles but also for individual nanomaterials, which is advantageous for the realization of high miniaturization and minimal invasiveness in biosensors and biodevices. Moreover, the nanomaterials' incorporations into biosensors have been shown to be useful and functional for a variety of optical detection modes, such as absorption, colorimetry, fluorescence, near-band-edge emission, deep-level emission, chemiluminescence, surface evanescent wave, whispering gallery mode, lossy-mode resonance, surface plasmon resonance, and surface-enhanced Raman scattering. The detection capabilities of these ZnO nanomaterial-based optical biosensors demonstrated so far are highly encouraging and, in some cases, permit quantitative analyses of ultra-trace level bioanalytes that cannot be measured by other means. Hence, steady research endeavors are expected in this burgeoning field, whose scientific and technological impacts will grow immensely in the future. This review provides a timely and much needed review of the research efforts made in the field of ZnO nanomaterial-based optical biosensors in a comprehensive and systematic manner. The topical discussions in this review are organized by the different modes of optical detection listed above and further grouped by the dimensionality of the ZnO nanostructures used in biosensors. Following an overview of a given optical detection mode, the unique properties of ZnO nanomaterials critical to enhanced biodetection are presented in detail. Subsequently, specific biosensing applications of ZnO nanomaterials are discussed for ~40 different bioanalytes, and the important roles that the ZnO nanomaterials play in bioanalyte detection are also identified.
Keywords: ZnO biosensors; ZnO nanomaterials; ZnO nanoparticles; ZnO nanorods; ZnO optical sensors; ZnO-enhanced biodetection; nanobiosensors; optical biodetection; optical biosensors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
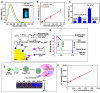
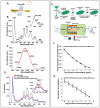
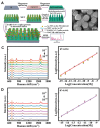
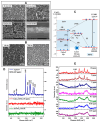
References
-
- Krishna M.S., Singh S., Batool M., Fahmy H.M., Seku K., Shalan A.E., Lanceros-Mendez S., Zafar M.N. A Review on 2D-ZnO Nanostructure Based Biosensors: From Materials to Devices. Mater. Adv. 2023;4:320–354. doi: 10.1039/D2MA00878E. - DOI
-
- Zhu P., Weng Z., Li X., Liu X., Wu S., Yeung K.W.K., Wang X., Cui Z., Yang X., Chu P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces. 2016;3:1500494. doi: 10.1002/admi.201500494. - DOI
-
- Escalona-Villalpando R.A., Viveros-Palma K., Espinosa-Lagunes F.I., Rodríguez-Morales J.A., Arriaga L.G., Macazo F.C., Minteer S.D., Ledesma-García J. Comparative Colorimetric Sensor Based on Bi-Phase γ-/α-Fe2O3 and γ-/α-Fe2O3/ZnO Nanoparticles for Lactate Detection. Biosensors. 2022;12:1025. doi: 10.3390/bios12111025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

