Non-Invasive On-Off Fluorescent Biosensor for Endothelial Cell Detection
- PMID: 39451702
- PMCID: PMC11506521
- DOI: 10.3390/bios14100489
Non-Invasive On-Off Fluorescent Biosensor for Endothelial Cell Detection
Abstract
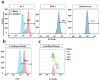
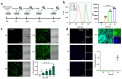
For rapid and convenient detection of living endothelial cells (ECs) specifically without immunostaining, we developed a biosensor based on turn-on fluorescent protein, named LV-EcpG. It includes a high-affinity peptide E12P obtained through phage display technology for specifically recognizing ECs and a turn-on EGFP fused with two linker peptides. The "on-off" switching mechanism of this genetically encoded fluorescent protein-based biosensor (FPB) ensured that fluorescence signals were activated only when binding with ECs, thus enabling these FPB characters for direct, visual, and non-invasive detection of ECs. Its specificity and multicolor imaging capability established LV-EcpG as a powerful tool for live EC research, with significant potential for diagnosing and treating cardiovascular diseases and tumor angiogenesis.
Keywords: affinity peptide; endothelial cells (ECs); genetically encoded fluorescent protein-based biosensors (FPBs); non-invasive; phage display.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Xing L., Huang G., Chen R., Huang L., Liu J., Ren X., Wang S., Kuang H., Kumar A., Kim J.K., et al. Critical role of mitogen-inducible gene 6 in restraining endothelial cell permeability to maintain vascular homeostasis. J. Cell Commun. Signal. 2023;17:151–165. doi: 10.1007/s12079-022-00704-z. - DOI - PMC - PubMed
-
- Zakharova I., Saaya S., Shevchenko A., Stupnikova A., Zhiven’ M., Laktionov P., Stepanova A., Romashchenko A., Yanshole L., Chernonosov A., et al. Mitomycin-treated endothelial and smooth muscle cells suitable for safe tissue engineering approaches. Front. Bioeng. Biotechnol. 2022;10:772981. doi: 10.3389/fbioe.2022.772981. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

