Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System
- PMID: 39451707
- PMCID: PMC11506760
- DOI: 10.3390/bios14100494
Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System
Abstract
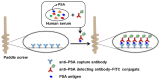
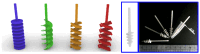
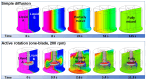
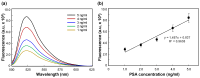
In this paper, we present a sensitive and highly reproducible fluorescence immunosensor for detecting PSA in human serum. A unique feature of this study is that it uses creatively designed paddle screw-type devices and their custom-made rotating system for PSA immunoassay. The paddle screw devices were designed to maximize the surface-to-volume ratio over which the immunoassay reaction could occur to improve detection sensitivity. This paddle screw-based immunoassay offers an accessible and efficient method with a short analysis time of less than 30 min. Active rotation of the paddle screw plays a crucial role in fast and accurate analysis of PSA. Additionally, a paddle screw-based immunoassay and subsequent fluorescence detection using a custom prototype fluorescence detection system were compared to a typical well plate-based immunoassay system. Results of PSA detection in human serum showed that the detection sensitivity through the paddle screw-based analysis improved about five times compared to that with a well plate-based analysis.
Keywords: 3D paddle screw; detection sensitivity; fluorescence measurement; immunoassay; prostate-specific antigen.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Koivunen M.E., Krogsrud R.L. Principles of immunochemical techniques used in clinical laboratories. Lab. Med. 2006;37:490–497. doi: 10.1309/MV9RM1FDLWAUWQ3F. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

