The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas
- PMID: 39451804
- PMCID: PMC11505355
- DOI: 10.3390/biomimetics9100598
The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas
Abstract
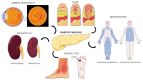
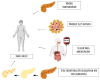
Type 1 diabetes mellitus (T1DM) is a chronic condition primarily managed with insulin replacement, leading to significant treatment costs. Complications include vasculopathy, cardiovascular diseases, nephropathy, neuropathy, and reticulopathy. Pancreatic islet transplantation is an option but its success does not depend solely on adequate vascularization. The main limitations to clinical islet transplantation are the scarcity of human pancreas, the need for immunosuppression, and the inadequacy of the islet isolation process. Despite extensive research, T1DM remains a major global health issue. In 2015, diabetes affected approximately 415 million people, with projected expenditures of USD 1.7 trillion by 2030. Pancreas transplantation faces challenges due to limited organ availability and complex vascularization. T1DM is caused by the autoimmune destruction of insulin-producing pancreatic cells. Advances in biomaterials, particularly the extracellular matrix (ECM), show promise in tissue reconstruction and transplantation, offering structural and regulatory functions critical for cell migration, differentiation, and adhesion. Tissue engineering aims to create bioartificial pancreases integrating insulin-producing cells and suitable frameworks. This involves decellularization and recellularization techniques to develop biological scaffolds. The challenges include replicating the pancreas's intricate architecture and maintaining cell viability and functionality. Emerging technologies, such as 3D printing and advanced biomaterials, have shown potential in constructing bioartificial organs. ECM components, including collagens and glycoproteins, play essential roles in cell adhesion, migration, and differentiation. Clinical applications focus on developing functional scaffolds for transplantation, with ongoing research addressing immunological responses and long-term efficacy. Pancreatic bioengineering represents a promising avenue for T1DM treatment, requiring further research to ensure successful implementation.
Keywords: decellularization; diabetes mellitus; extracellular matrix; insulin-producing cells; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Assessing Implantation Sites for Pancreatic Islet Cell Transplantation: Implications for Type 1 Diabetes Mellitus Treatment.Bioengineering (Basel). 2025 May 9;12(5):499. doi: 10.3390/bioengineering12050499. Bioengineering (Basel). 2025. PMID: 40428118 Free PMC article. Review.
-
Tissue engineering of decellularized pancreas scaffolds for regenerative medicine in diabetes.Acta Biomater. 2023 Feb;157:49-66. doi: 10.1016/j.actbio.2022.11.032. Epub 2022 Nov 24. Acta Biomater. 2023. PMID: 36427686 Review.
-
Comparative Decellularization and Recellularization of Normal Versus Streptozotocin-Induced Diabetes Mellitus Rat Pancreas.Artif Organs. 2019 Apr;43(4):399-412. doi: 10.1111/aor.13353. Epub 2018 Oct 28. Artif Organs. 2019. PMID: 30182423
-
Donor age significantly influences the Raman spectroscopic biomolecular fingerprint of human pancreatic extracellular matrix proteins following collagenase-based digestion.Acta Biomater. 2019 Nov;99:269-283. doi: 10.1016/j.actbio.2019.09.013. Epub 2019 Sep 13. Acta Biomater. 2019. PMID: 31525537
-
Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture.J Tissue Eng Regen Med. 2018 May;12(5):1230-1237. doi: 10.1002/term.2655. Epub 2018 Apr 10. J Tissue Eng Regen Med. 2018. PMID: 29499099
Cited by
-
Assessing Implantation Sites for Pancreatic Islet Cell Transplantation: Implications for Type 1 Diabetes Mellitus Treatment.Bioengineering (Basel). 2025 May 9;12(5):499. doi: 10.3390/bioengineering12050499. Bioengineering (Basel). 2025. PMID: 40428118 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

