Heterogeneous Nuclear Ribonucleoprotein A1 Knockdown Alters Constituents of Nucleocytoplasmic Transport
- PMID: 39452051
- PMCID: PMC11505608
- DOI: 10.3390/brainsci14101039
Heterogeneous Nuclear Ribonucleoprotein A1 Knockdown Alters Constituents of Nucleocytoplasmic Transport
Abstract
Background/objectives: Changes in nuclear morphology, alterations to the nuclear pore complex (NPC), including loss, aggregation, and dysfunction of nucleoporins (Nups), and nucleocytoplasmic transport (NCT) abnormalities have become hallmarks of neurodegenerative diseases. Previous RNA sequencing data utilizing knockdown of heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) identified enrichment for pathways and changes in RNAs related to nuclear morphology and showed differential expression of key nuclear targets. This suggests that dysfunction of hnRNP A1, which is observed in neurodegenerative diseases, may contribute to abnormalities in nuclear morphology, NPC, and NCT.
Methods: We performed knockdown of hnRNP A1 in Neuro-2A cells, a neuronal cell line, to examine nuclear morphology, NPC, and NCT.
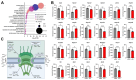
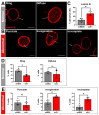
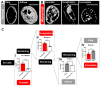
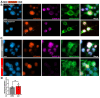
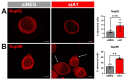
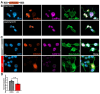
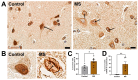
Results: First, we examined nuclear morphology using Lamin B, wherein we observed increased nuclear envelope abnormalities in cells with hnRNP A1 knockdown as compared to control. To quantify changes in Lamin B, we designed and validated an automated computer-based model, which quantitatively confirmed our observations. Next, we investigated the impact of hnRNP A1 knockdown on components of the NPC and NCT. In line with the previous literature, we found changes in Nups, including altered distribution and reduced protein expression, as well as disrupted NCT. Finally, we validated our findings in multiple sclerosis (MS) brains, a disease with a significant neurodegenerative component caused by hnRNP A1 dysfunction, where neuronal nuclear envelope alterations were significantly increased as compared to controls.
Conclusions: Together, these data implicate hnRNP A1 as an important contributor to nuclear morphology, Nup expression and distribution, and NCT and suggest that hnRNP A1 dysfunction may lead to defects in these processes in neurodegenerative diseases.
Keywords: RNA binding protein; hnRNP A1; nuclear pore complex; nucleocytoplasmic transport.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Heterogeneous Nuclear Ribonucleoprotein A1 and Lamin A/C Modulate Nucleocytoplasmic Shuttling of Avian Reovirus p17.J Virol. 2019 Sep 30;93(20):e00851-19. doi: 10.1128/JVI.00851-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375578 Free PMC article.
-
Knock-Down of Heterogeneous Nuclear Ribonucleoprotein A1 Results in Neurite Damage, Altered Stress Granule Biology, and Cellular Toxicity in Differentiated Neuronal Cells.eNeuro. 2021 Nov 18;8(6):ENEURO.0350-21.2021. doi: 10.1523/ENEURO.0350-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34697074 Free PMC article.
-
hnRNP A1 dysfunction in oligodendrocytes contributes to the pathogenesis of multiple sclerosis.Glia. 2023 Mar;71(3):633-647. doi: 10.1002/glia.24300. Epub 2022 Nov 16. Glia. 2023. PMID: 36382566
-
Heterogeneous nuclear ribonucleoprotein A1 in health and neurodegenerative disease: from structural insights to post-transcriptional regulatory roles.Mol Cell Neurosci. 2013 Sep;56:436-46. doi: 10.1016/j.mcn.2012.12.002. Epub 2012 Dec 14. Mol Cell Neurosci. 2013. PMID: 23247072 Review.
-
A Comprehensive Analysis of the Role of hnRNP A1 Function and Dysfunction in the Pathogenesis of Neurodegenerative Disease.Front Mol Biosci. 2021 Apr 12;8:659610. doi: 10.3389/fmolb.2021.659610. eCollection 2021. Front Mol Biosci. 2021. PMID: 33912591 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

