Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats
- PMID: 39452068
- PMCID: PMC11505095
- DOI: 10.3390/biology13100759
Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats
Abstract
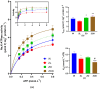
Type 2 diabetes mellitus has been referred to as being closely related to oxidative stress, which may affect brain functions and brain glucose metabolism due to its high metabolic activity and lipid-rich content. Na,K-ATPase is an essential enzyme maintaining intracellular homeostasis, with properties that can sensitively mirror various pathophysiological conditions such as diabetes. The goal of this study was to determine oxidative stress markers as well as Na,K-ATPase activities in the cerebellum of Zucker diabetic fatty (ZDF) rats depending on diabetes severity. The following groups of male rats were used: Wistar, ZDF Lean (fa/+), and ZDF (fa/fa) rats, arbitrarily divided according to glycemia into ZDF obese (ZO, less severe diabetes) and ZDF diabetic (ZOD, advanced diabetes) groups. In addition to basic biometry and biochemistry, oxidative stress markers were assessed in plasma and cerebellar tissues. The Na, K-ATPase enzyme activity was measured at varying ATP substrate concentrations. The results indicate significant differences in basic biometric and biochemical parameters within all the studied groups. Furthermore, oxidative damage was greater in the cerebellum of both ZDF (fa/fa) groups compared with the controls. Interestingly, Na,K-ATPase enzyme activity was highest to lowest in the following order: ZOD > ZO > Wistar > ZDF lean rats. In conclusion, an increase in systemic oxidative stress resulting from diabetic conditions has a significant impact on the cerebellar tissue independently of diabetes severity. The increased cerebellar Na,K-ATPase activity may reflect compensatory mechanisms in aged ZDF (fa/fa) animals, rather than indicating cerebellar neurodegeneration: a phenomenon that warrants further investigation.
Keywords: Na,K-ATPase enzyme; Zucker diabetic fatty rats; cerebellum; diabetes severity; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ogurtsova K., Da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

