Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite
- PMID: 39452078
- PMCID: PMC11504028
- DOI: 10.3390/biology13100769
Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite
Abstract
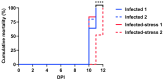
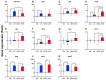
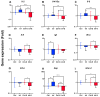
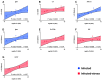
Farmed fish are regularly subjected to various stressors due to farming practices, and their effect in the context of a disease outbreak is uncertain. This research evaluated the effects of unpredictable repeated stress in rainbow trout challenged with the ciliate Ichthyophthirius multifiliis, known to cause white spot disease in freshwater fish. Before and after the pathogen exposure, fish were handled with a random rotation of three procedures. At 7 days post-infection (dpi), the parasite burden was evaluated in fish and in the tank's water, and the local and systemic immune responses were investigated in the gill and spleen, respectively. The fish mortality was recorded until 12 dpi, when all the fish from the infected groups died. There was no statistical difference in parasite burden (fish and tank's water) and infection severity between the two infected fish groups. The immune gene expression analysis suggested a differential immune response between the gill and the spleen. In gills, a T helper cell type 2 immune response was initiated, whereas in spleen, a T helper cell type 1 immune response was observed. The stress has induced mainly upregulations of immune genes in the gill (cat-1, hep, il-10) and downregulations in the spleen (il-2, il-4/13a, il-8). Our results suggested that the unpredictable repeated stress protocol employed did not impair the fish immune system.
Keywords: Ichthyophthirius multifiliis; fish handling; immune gene expression; immune signaling; parasite infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- MacColl E., Therkelsen M.D., Sherpa T., Ellerbrock H., Johnston L.A., Jariwala R.H., Chang W., Gurtowski J., Schatz M.C., Hossain M.M., et al. Molecular genetic diversity and characterization of conjugation genes in the fish parasite Ichthyophthirius multifiliis. Mol. Phylogenet. Evol. 2015;86:1–7. doi: 10.1016/j.ympev.2015.02.017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

