Phytochemistry and Evaluation of the Anti-Inflammatory Activity of the Hydroethanolic Extract of Virola elongata (Benth.) Warb. Stem Bark
- PMID: 39452085
- PMCID: PMC11505066
- DOI: 10.3390/biology13100776
Phytochemistry and Evaluation of the Anti-Inflammatory Activity of the Hydroethanolic Extract of Virola elongata (Benth.) Warb. Stem Bark
Abstract
Background: Previous studies of the hydroethanolic extract of Virola elongata inner stem bark (HEVe) have demonstrated its antioxidant, gastroprotective, and antiulcer properties, but have not evaluated its anti-inflammatory potential.
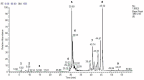
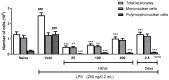
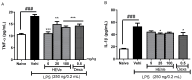
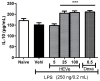
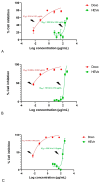
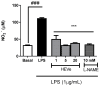
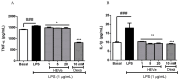
Methods: HEVe was obtained by maceration and phytochemically analyzed. Its systemic anti-inflammatory activity was assessed by its effect on lipopolysaccharide (LPS)-induced peritonitis in mice. HEVe gel (HEgVe) was employed to evaluate topical anti-inflammatory activity by measuring the ear edema resulting from croton-oil-induced dermatitis in mice. A cell viability assay was conducted to determine the non-cytotoxic concentrations of the HEVe. RAW 264.7 cells were stimulated by LPS to determinate cytokine and nitric oxide production.
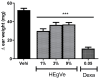
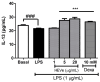
Results: A phytochemical analysis of the HEVe revealed the presence of phenolic acids, neolignans, flavonoids, and monomeric catechins. The oral treatment of acute peritonitis with HEVe reduced the total leukocytes, neutrophils, TNF-α, and IL-1β and elevated IL-10 levels. The application of the HEgVe reduced local edema. The HEVe on the RAW 264.7 cells exhibited no cytotoxicity, and the cells with HEVe displayed reduced TNF-α, IL-1β, and NO levels and increased IL-13 levels.
Conclusions: HEVe demonstrated systemic and topical multitarget anti-inflammatory activity, likely due to the combined effects of secondary metabolites. HEVe emerges as a promising herbal remedy for inflammation with minimal cytotoxicity, emphasizing its potential therapeutic significance.
Keywords: Virola elongata; cytotoxicity; extract; inflammation; leukocytes; phytochemistry.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Chow C.W., Downey G.P. Basic biology and critical care medicine—Inflammation. In: Albert R.K., Slutsky A.S., Ranieri V.M., Torres A., Takala J., editors. Clinical Critical Care Medicine. 1st ed. Volume 1. Elsevier Inc.; Grand Rapids, MI, USA: 2006. pp. 1–12.
Grants and funding
LinkOut - more resources
Full Text Sources

