Activity of Organoboron Compounds against Biofilm-Forming Pathogens
- PMID: 39452196
- PMCID: PMC11504661
- DOI: 10.3390/antibiotics13100929
Activity of Organoboron Compounds against Biofilm-Forming Pathogens
Abstract
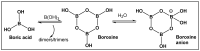
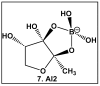
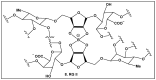
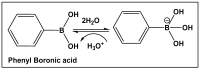
Bacteria have evolved and continue to change in response to environmental stressors including antibiotics. Antibiotic resistance and the ability to form biofilms are inextricably linked, requiring the continuous search for alternative compounds to antibiotics that affect biofilm formation. One of the latest drug classes is boron-containing compounds. Over the last several decades, boron has emerged as a prominent element in the field of medicinal chemistry, which has led to an increasing number of boron-containing compounds being considered as potential drugs. The focus of this review is on the developments in boron-containing organic compounds (BOCs) as antimicrobial/anti-biofilm probes and agents.
Keywords: anti-biofilm; antimicrobial; boron-containing compounds; inhibitors of pathogenic bacterial enzymes; multidrug resistance; phenotypic screens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


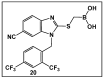
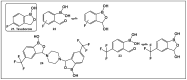
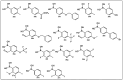

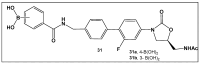





References
-
- Templeton D.M., Ariese F., Cornelis R., Danielsson L.-G., Muntau H., van Leeuwen H.P., Lobinski R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000) Pure Appl. Chem. 2000;72:1453–1470. doi: 10.1351/pac200072081453. - DOI
Publication types
LinkOut - more resources
Full Text Sources

