Investigation of the Impact of Antibiotic Administration on the Preterm Infants' Gut Microbiome Using Next-Generation Sequencing-Based 16S rRNA Gene Analysis
- PMID: 39452243
- PMCID: PMC11505465
- DOI: 10.3390/antibiotics13100977
Investigation of the Impact of Antibiotic Administration on the Preterm Infants' Gut Microbiome Using Next-Generation Sequencing-Based 16S rRNA Gene Analysis
Abstract
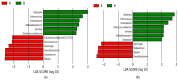
Background: The human gut microbiota is an extensive population of microorganisms, and it shows significant variations between periods of optimal health and periods of illness. Vancomycin-resistant Enterococcus (VRE) and carbapenem-resistant Klebsiella pneumoniae (CRKP) are both pathogenic agents (BPAs) that can colonize in the gut after dysbiosis of microbiotal composition following antibiotic treatment. Methods: This study aimed to investigate the impact of antibiotics on the microbiotal composition of the gut. For this purpose, the first pass meconiums of 20 patients and the first rectal swabs containing BPAs of the same patients after antibiotic treatment were studied using next-generation sequencing-based 16S rRNA gene analysis. The V1-V9 region of 16S rRNA was sequenced with Oxford Nanopore. Results: Twenty-five phyla were detected in the meconiums, and 12 of them were absent after antibiotic treatment. The four most prevalent phyla in meconiums were Bacillota, Pseudomonadota, Bacteroidota, and Actinomycetota. Only the relative abundance of Pseudomonadota was increased, while a significant decrease was observed in the other three phyla (p < 0.05). A significant decrease was observed in alpha-diversity in rectal swabs containing BPAs versus meconiums (p = 0.00408), whereas an increased variance was observed in beta-diversity in all samples (p < 0.05). As a result of a LEfSe analysis, Pseudomonadota was found to have a higher relative abundance in rectal swabs, and Bacillota was significantly higher in the meconiums of the twins. Conclusions: Our study strongly verified the relationship between the administration of antibiotics, dysbiosis, and colonization of BPAs in the infants' gut microbiota. Further research would be beneficial and needed, comprising the natural development process of the infants' gut microbiota.
Keywords: 16S rRNA; antibiotic; carbapenem-resistant Klebsiella pneumoniae; meconium; microbiome; microbiota; preterm infants; vancomycin-resistant Enterococcus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

