Pharmacokinetics of Vancomycin in Healthy Korean Volunteers and Monte Carlo Simulations to Explore Optimal Dosage Regimens in Patients with Normal Renal Function
- PMID: 39452259
- PMCID: PMC11504268
- DOI: 10.3390/antibiotics13100993
Pharmacokinetics of Vancomycin in Healthy Korean Volunteers and Monte Carlo Simulations to Explore Optimal Dosage Regimens in Patients with Normal Renal Function
Abstract
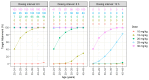
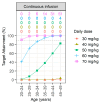
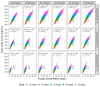
Background/Objectives: To date, population pharmacokinetic (PK) studies of vancomycin on healthy Korean adults have not been conducted. This study aimed to investigate the PK properties of vancomycin in healthy volunteers and to identify optimal dosing regimens based on the area under the concentration-time curve (AUC) in adult patients with normal renal function. Methods: We conducted a prospective clinical study, analysing PK samples from 12 healthy participants using noncompartmental analysis and non-linear mixed-effects modelling. The population PK parameters derived were employed in Monte Carlo simulations to evaluate the adequacy of the current dosing regimen and to formulate dosing recommendations. Results: The PK profiles were optimally described by a two-compartment model, with body weight and age as significant covariates affecting total clearance. The simulations indicated that to achieve a therapeutic target-defined as an AUC at steady-state over 24 h of 400-600 mg·h/L-daily doses ranging from 60 to 70 mg/kg are necessary in adults with normal renal function. Conclusions: This study underscores the need to actively adjust dosage and administration based on a vancomycin PK model that adequately reflects the demographic characteristics of patients to meet both safety and efficacy standards.
Keywords: Monte Carlo simulation; adult; healthy; normal renal function; population pharmacokinetics; vancomycin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Hasanpour A.H., Sepidarkish M., Mollalo A., Ardekani A., Almukhtar M., Mechaal A., Hosseini S.R., Bayani M., Javanian M., Rostami A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2023;12:4. doi: 10.1186/s13756-023-01210-6. - DOI - PMC - PubMed
-
- Lee H., Yoon E.J., Kim D., Jeong S.H., Won E.J., Shin J.H., Kim S.H., Shin J.H., Shin K.S., Kim Y.A., et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: First one-year report from Kor-GLASS. Eurosurveillance. 2018;23:1800047. doi: 10.2807/1560-7917.ES.2018.23.42.1800047. - DOI - PMC - PubMed
-
- Rybak M.J., Le J., Lodise T.P., Levine D.P., Bradley J.S., Liu C., Mueller B.A., Pai M.P., Wong-Beringer A., Rotschafer J.C., et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2020;77:835–864. doi: 10.1093/ajhp/zxaa036. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

