Storytelling of Hypertrophic Cardiomyopathy Discovery
- PMID: 39452271
- PMCID: PMC11508572
- DOI: 10.3390/jcdd11100300
Storytelling of Hypertrophic Cardiomyopathy Discovery
Abstract
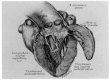
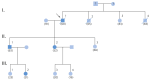
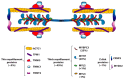
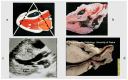
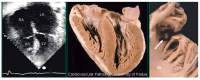
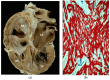
The discovery of hypertrophic cardiomyopathy (HCM) dates back to 1958, when the pathologist Donald Teare of the St. George's Hospital in London performed autopsies in eight cases with asymmetric hypertrophy of the ventricular septum and bizarre disorganization (disarray) at histology, first interpreted as hamartoma. Seven had died suddenly. The cardiac specimens were cut along the long axis, similar to the 2D echo. In the same year, at the National Institute of Health U.S.A., Eugene Braunwald, a hemodynamist, and Andrew Glenn Morrow, a cardiac surgeon, clinically faced a patient with an apparently similar morbid entity, with a systolic murmur and subaortic valve gradient. "Discrete" subaortic stenosis was postulated. However, at surgery, Dr. Morrow observed only hypertrophy and performed myectomy to relieve the obstruction. This first Braunwald-Morrow patient underwent a successful cardiac transplant later at the disease end stage. The same Dr. Morrow was found to be affected by the familial HCM and died suddenly in 1992. The term "functional subaortic stenosis" was used in 1959 and "idiopathic hypertrophic subaortic stenosis" in 1960. Years before, in 1957, Lord Brock, a cardiac surgeon at the Guy's Hospital in London, during alleged aortic valve surgery in extracorporeal circulation, did not find any valvular or discrete subaortic stenoses. In 1980, John F. Goodwin of the Westminster Hospital in London, the head of an international WHO committee, put forward the first classification of heart muscle diseases, introducing the term cardiomyopathy (dilated, hypertrophic, and endomyocardial restrictive). In 1995, the WHO classification was revisited, with the addition of two new entities, namely arrhythmogenic and purely myocardial restrictive, the latter a paradox of a small heart accounting for severe congestive heart failure by ventricular diastolic impairment. A familial occurrence was noticed earlier in HCM and published by Teare and Goodwin in 1960. In 1989-1990, the same family underwent molecular genetics investigation by the Seidman team in Boston, and a missense mutation of the β-cardiac myosin heavy chain in chromosome 14 was found. Thus, 21 years elapsed from HCM gross discovery to molecular discoveries. The same original family was the source of both the gross and genetic explanations of HCM, which is now named sarcomere disease. Restrictive cardiomyopathy, characterized grossly without hypertrophy and histologically by myocardial disarray, was found to also have a sarcomeric genetic mutation, labeled "HCM without hypertrophy". Sarcomere missense mutations have also been reported in dilated cardiomyopathy (DCM) and non-compaction cardiomyopathy. Moreover, sarcomeric gene defects have been detected in some DNA non-coding regions of HCM patients. The same mutation in the family may express different phenotypes (HCM, DCM, and RCM). Large ischemic scars have been reported by pathologists and are nowadays easily detectable in vivo by cardiac magnetic resonance with gadolinium. The ischemic arrhythmic substrate enhances the risk of sudden death.
Keywords: cardiomyopathies; history of medicine; hypertrophic cardiomyopathy; pathology; restrictive cardiomyopathy; sudden cardiac death; transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures















References
-
- Aygen M.M., Hilbish T.F., Braunwald E., Morrow A.G., Cornell W.P. Idiopathic hypertrophic subaortic stenosis: Clinical, hemodynamic and angiographic manifestations. Am. J. Med. 1960;29:924–945.
-
- Brock R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp. Rep. 1957;106:221–238. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

