Therapeutic Drug Monitoring of Elexacaftor, Tezacaftor, and Ivacaftor in Adult People with Cystic Fibrosis
- PMID: 39452571
- PMCID: PMC11508966
- DOI: 10.3390/jpm14101065
Therapeutic Drug Monitoring of Elexacaftor, Tezacaftor, and Ivacaftor in Adult People with Cystic Fibrosis
Abstract
Background/objectives: Elexacaftor, tezacaftor, and ivacaftor (ETI) have significantly improved lung function in people with cystic fibrosis (pwCF). Despite exceptional improvements in most cases, treatment-related inter-subject variability and drug-drug interactions that complicate modulator therapy have been reported.
Methods: This retrospective analysis presents data on the serum concentration of ETI in our pwCF with full or reduced dosage from August 2021 to December 2023 via routine therapeutic drug monitoring (TDM). The data were compared with the maximum drug concentrations (Cmax) from the pharmaceutical company's summary of product characteristics.
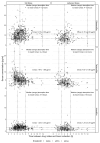
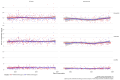
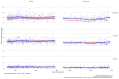
Results: A total of 786 blood samples from 155 pwCF (41% female, 59% male) were analyzed. The examinations were divided into four groups: full dose within the given tmax (38.5% of all measurements), full dose outside the tmax (29%), reduced dose within the tmax (19.2%), and reduced dose outside the tmax (13.2%). In pwCF receiving the full dose and blood taken within the tmax, 45.3% of serum concentrations of elexacaftor, 51.1% of serum concentrations of ivacaftor, and 8.9% of serum concentrations of tezacaftor were found to be above the Cmax, respectively. For those on reduced doses within the tmax, 24.5% had a serum concentration of elexacaftor, 23.2% had a serum concentration of ivacaftor, and 2.5% had a serum concentration of tezacaftor above the Cmax, respectively.
Conclusions: Many pwCF under ETI therapy have Cmax values for elexacaftor and ivacaftor above the recommended range, even on reduced doses or before the tmax was reached. This highlights the value of a TDM program. Further pharmacokinetic studies are necessary.
Keywords: cystic fibrosis; elexacaftor; ivacaftor; serum concentrations; tezacaftor; therapeutic drug monitoring.
Conflict of interest statement
SN reports a CTN-ECFS grant (for staff for clinical studies) as well as one speaker’s fee from Vertex. SS and JK report receiving a fee for the statistical analysis. OS reports grants from the Deutsche Zentrum für Lungenforschung (DZL) and payments from Vertex Pharmaceuticals for clinical trials and lectures. UL reports a grant from the German research foundation and from Else-Kröner Fresenius Stiftung, consulting fees from CytoSorbents Europe GmbH, fees for advisory boards for Roche International Ltd., as well as various travel grants to meetings from KAIMS and DGAI. SS and JK are staff members of STAT-UP Statistical Consulting & Data Science GmbH; they have conducted the statistical analysis. The remaining authors do not have a financial relationship that creates or may be perceived as creating a conflict related to this article.
Figures
References
-
- Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., Ramsey B.W., Taylor-Cousar J.L., Tullis E., Vermeulen F., et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. - DOI - PMC - PubMed
-
- Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., Mall M.A., Welter J.J., Ramsey B.W., McKee C.M., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

