Prophylactic Administration of Perampanel for Post-Stroke Epilepsy (PROPELLER Study): A Trial Protocol
- PMID: 39452793
- PMCID: PMC11510630
- DOI: 10.3390/mps7050079
Prophylactic Administration of Perampanel for Post-Stroke Epilepsy (PROPELLER Study): A Trial Protocol
Abstract
Background: Post-stroke epilepsy can reduce patients' abilities to carry out various activities of daily living. Despite their importance in preventing the onset of post-stroke epilepsy, the prophylactic administration of antiepileptic drugs is controversial due to a lack of high-level clinical research. In this study, we initiated a prospective interventional study of prophylactic antiepileptic drug administration in patients with a subcortical hemorrhage, who are at the highest risk of developing epilepsy after experiencing a stroke.
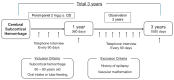
Methods: The study was conducted in a single-center setting and was a single-arm study with no control group; the case entry period started in November 2023 and is due to end in March 2025. Only cases with a subcortical hemorrhage will be included. The treatment regimen used in this study is 2 mg of perampanel per day. Perampanel will be administered for one year, followed by two years of follow-up, for a total study period of three years. The primary endpoint will be the development of epilepsy.
Results: Perampanel administration is expected to reduce the incidence of post-stroke epilepsy in comparison to the results of previous reports on the use of alternative treatments.
Conclusions: The results of this study will provide new insights into the prevention of post-stroke epilepsy. The relatively small size of this study makes it difficult to provide strong evidence of the efficacy of perampanel, but it may serve as a basis for larger clinical trials.
Keywords: perampanel; post-stroke epilepsy; prophylactic treatment; prospective interventional study.
Conflict of interest statement
The authors declare that they have no competing interests.
Eisai (Eisai Co., Ltd. Tokyo, Japan), which produces and distributes perampanel in Japan, provided our hospital with nonfinancial support.
The authors declare no conflicts of interest.
References
Grants and funding
LinkOut - more resources
Full Text Sources