Phycocyanin-Loaded Alginate-Based Hydrogel Synthesis and Characterization
- PMID: 39452842
- PMCID: PMC11509733
- DOI: 10.3390/md22100434
Phycocyanin-Loaded Alginate-Based Hydrogel Synthesis and Characterization
Abstract
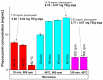
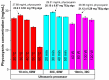
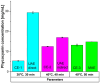
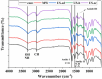
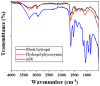
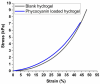
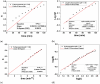
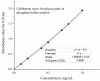
Phycocyanin was extracted from Spirulina platensis using conventional extraction (CE), direct ultrasonic-assisted extraction (direct UAE), indirect ultrasonic-assisted extraction (indirect UAE), and microwave-assisted extraction (MAE) methods at different temperatures, extraction intervals, stirring rate, and power intensities while maintaining the same algae to solvent ratio (1:15 w/v). The optimization of the extraction parameters indicated that the direct UAE yielded the highest phycocyanin concentration (29.31 ± 0.33 mg/mL) and antioxidant activity (23.6 ± 0.56 mg TE/g algae), while MAE achieved the highest purity (Rp = 0.5 ± 0.002). Based on the RP value, phycocyanin extract obtained by MAE (1:15 w/v algae to solvent ratio, 40 min, 40 °C, and 900 rpm) was selected as active compound in an alginate-based hydrogel formulation designed as potential wound dressings. Phycocyanin extracts and loaded hydrogels were characterized by FT-IR analysis. SEM analysis confirmed a porous structure for both blank and phycocyanin loaded hydrogels, while the mechanical properties remained approximately unchanged in the presence of phycocyanin. Phycocyanin release kinetics was investigated at two pH values using Zero-order, First-order, Higuchi, and Korsmeyer-Peppas kinetics models. The Higuchi model best fitted the experimental results. The R2 value at higher pH was nearly 1, indicating a superior fit compared with lower pH values.
Keywords: Spirulina platensis; microwave extraction; phycocyanin extract; release mechanism; ultrasonic extraction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Tomaselli L. Morphology, Ultrastructure and Taxonomy of Arthrospira (Spirulina) maxima and Arthrospira (Spirulina) plantesis. In: Vonshak A., editor. Spirulina Plantesis (Arthospira) Physiology, Cell-Biology and Biotechnology. Taylor & Francis Ltd.; London, UK: 2002. pp. 1–16.
-
- Masojídek J., Torzillo G. Mass Cultivation of Freshwater Microalgae. In: Jørgensen S.E., Fath B.D., editors. Encyclopedia of Ecology. Academic Press; Oxford, UK: 2008. pp. 2226–2235.
-
- Ho J.N., Watson R.R., Lee J. Chapter 11—Dietary Supplements, Immune Modulation, and Diabetes Control. In: Watson R.R., Preedy V.R., editors. Bioactive Food as Dietary Interventions for Diabetes. Academic Press; San Diego, CA, USA: 2013. pp. 111–120.
-
- Taufiqurrahmi N., Religia P., Mulyani G., Suryana D., Ichsan I., Tanjung F., Arifin Y. Phycocyanin extraction in Spirulina produced using agricultural waste. IOP Conf. Ser. Mater. Sci. Eng. 2017;206:012097. doi: 10.1088/1757-899X/206/1/012097. - DOI
-
- Brito A.d.F., Silva A.S., de Oliveira C.V.C., de Souza A.A., Ferreira P.B., de Souza I.L.L., da Cunha Araujo L.C., da Silva Félix G., de Souza Sampaio R., Tavares R.L., et al. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: Dose-response relation study. Sci. Rep. 2020;10:6382. doi: 10.1038/s41598-020-63272-5. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- PN-III-P2-2.1-PED-2021-ctr.no. 672PED/2022/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PN 2307/29.12.2022 within PNCDI IV/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- PubArt Program/National University of Science and Technology POLITEHNICA Bucharest
LinkOut - more resources
Full Text Sources

