Exploring the Physicochemical Characteristics of Marine Protein Hydrolysates and the Impact of In Vitro Gastrointestinal Digestion on Their Bioactivity
- PMID: 39452860
- PMCID: PMC11509636
- DOI: 10.3390/md22100452
Exploring the Physicochemical Characteristics of Marine Protein Hydrolysates and the Impact of In Vitro Gastrointestinal Digestion on Their Bioactivity
Abstract
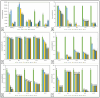
Fish protein hydrolysates (FPHs) were obtained from different fish sources using a combination of microbial enzymes. The industrially produced FPHs from blue whiting (Micromesistius poutassou) and sprat (Sprattus sprattus) were compared to freeze-dried FPHs generated in-house from hake (Merluccius merluccius) and mackerel (Scomber scombrus) in terms of their physicochemical composition and functionality. Significant differences (p < 0.05) were observed in the protein, moisture, and ash contents of the FPHs, with the majority having high levels of protein (73.24-89.31%). Fractions that were more extensively hydrolysed exhibited a high solubility index (74.05-98.99%) at different pHs. Blue whiting protein hydrolysate-B (BWPH-B) had the highest foaming capacity at pH 4 (146.98 ± 4.28%) and foam stability over 5 min (90-100%) at pH 4, 6, and 8. The emulsifying capacity ranged from 61.11-108.90 m2/g, while emulsion stability was 37.82-76.99% at 0.5% (w/v) concentration. In terms of peptide bioactivity, sprat protein hydrolysate (SPH) had the strongest overall reducing power. The highest Cu2+ chelating activity was exhibited by hake protein hydrolysate (HPH) and mackerel protein hydrolysate (MPH), with IC50 values of 0.66 and 0.78 mg protein/mL, respectively, while blue whiting protein hydrolysate-A (BWPH-A) had the highest activity against Fe2+ (IC50 = 1.89 mg protein/mL). SPH scavenged DPPH and ABTS radicals best with IC50 values of 0.73 and 2.76 mg protein/mL, respectively. All FPHs displayed noteworthy scavenging activity against hydroxyl radicals, with IC50 values ranging from 0.48-3.46 mg protein/mL. SPH and MPH showed the highest scavenging potential against superoxide radicals with IC50 values of 1.75 and 2.53 mg protein/mL and against hydrogen peroxide with 2.22 and 3.66 mg protein/mL, respectively. While inhibition of α-glucosidase was not observed, the IC50 values against α-amylase ranged from 8.81-18.42 mg protein/mL, with SPH displaying the highest activity. The stability of FPHs following simulated gastrointestinal digestion (SGID) showed an irregular trend. Overall, the findings suggest that marine-derived protein hydrolysates may serve as good sources of natural nutraceuticals with antioxidant and antidiabetic properties.
Keywords: amino acids; antioxidant activity; bioactive peptides; diabetes; fish protein hydrolysates; functional properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Tadesse S.A., Emire S.A., Barea P., Illera A.E., Melgosa R., Beltrán S., Sanz M.T. Valorisation of low-valued ray-finned fish (Labeobarbus nedgia) by enzymatic hydrolysis to obtain fish-discarded protein hydrolysates as functional foods. Food Bioprod. Process. 2023;141:167–184. doi: 10.1016/j.fbp.2023.08.003. - DOI
-
- Alahmad K., Noman A., Xia W., Jiang Q., Xu Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis) Molecules. 2023;28:519. doi: 10.3390/molecules28020519. - DOI - PMC - PubMed
-
- Wu M.-F., Xi Q.-H., Sheng Y., Wang Y.-M., Wang W.-Y., Chi C.-F., Wang B. Antioxidant Peptides from Monkfish Swim Bladders: Ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway. Mar. Drugs. 2023;21:360. doi: 10.3390/md21060360. - DOI - PMC - PubMed
-
- Henriques A., Vázquez J.A., Valcarcel J., Mendes R., Bandarra N.M., Pires C. Characterization of protein hydrolysates from fish discards and by-products from the north-west Spain fishing fleet as potential sources of bioactive peptides. Mar. Drugs. 2021;19:338. doi: 10.3390/md19060338. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

