Potential of Marine Sponge Metabolites against Prions: Bromotyrosine Derivatives, a Family of Interest
- PMID: 39452864
- PMCID: PMC11509309
- DOI: 10.3390/md22100456
Potential of Marine Sponge Metabolites against Prions: Bromotyrosine Derivatives, a Family of Interest
Abstract
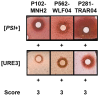
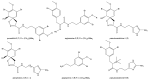
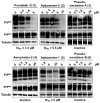
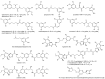
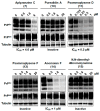
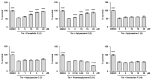
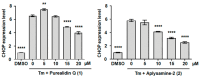
The screening of 166 extracts from tropical marine organisms (invertebrates, macroalgae) and 3 cyclolipopeptides from microorganisms against yeast prions highlighted the potential of Verongiida sponges to prevent the propagation of prions. We isolated the known compounds purealidin Q (1), aplysamine-2 (2), pseudoceratinine A (3), aerophobin-2 (4), aplysamine-1 (5), and pseudoceratinine B (6) for the first time from the Wallisian sponge Suberea laboutei. We then tested compounds 1-6 and sixteen other bromotyrosine and bromophenol derivatives previously isolated from Verongiida sponges against yeast prions, demonstrating the potential of 1-3, 5, 6, aplyzanzine C (7), purealidin A (10), psammaplysenes D (11) and F (12), anomoian F (14), and N,N-dimethyldibromotyramine (15). Following biological tests on mammalian cells, we report here the identification of the hitherto unknown ability of the six bromotyrosine derivatives 1, 2, 5, 7, 11, and 14 of marine origin to reduce the spread of the PrPSc prion and the ability of compounds 1 and 2 to reduce endoplasmic reticulum stress. These two biological activities of these bromotyrosine derivatives are, to our knowledge, described here for the first time, offering a new therapeutic perspective for patients suffering from prion diseases that are presently untreatable and consequently fatal.
Keywords: ER stress; PrPSc; Suberea laboutei; Verongiida; bromotyrosine derivatives; marine sponges; prion diseases; yeast-based screening.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
- Proteomar project/Institut Universitaire Européen de la Mer - Univ Brest
- Proteomar project/Institut Brestois Santé Agro Matière - Univ Brest
- Wallis 2018 cruise/Flotte Océanographique Française
- Wallis 2018 cruise/Labex Mer
- GGB Laboratory (UMR1078)/Inserm Grand Ouest
- LEMAR (UMR6539)/Institut de Recherche pour le Développement
- Maha Sinane PhD thesis/Ministère de l'enseignement supérieur et de la recherche
- Lucile Gentile PhD thesis/Ministère de l'enseignement supérieur et de la recherche
- Colin Grunberger Master 1 and Master 2 thesis/Institut de Recherche pour le Développement
- ICSN/Centre National de la Recherche Scientifique
- Wallis 2018 cruise/Institut de Recherche pour le Développement
- Wallis 2018 cruise/Museum National d'Histoire Naturelle
- Wallis 2018 cruise/Wallis and Futuna Environment Service
- GGB Laboratory (UMR1078) - Group PRiME/NRJ-Institut de France
- GGB Laboratory (UMR1078) - Group PRiME/Comité d'entente et de coordination des associations de Parkinsoniens (CECAP)
LinkOut - more resources
Full Text Sources
Research Materials

