Novel Insights into Ethanol-Soluble Oyster Peptide-Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells
- PMID: 39452873
- PMCID: PMC11509544
- DOI: 10.3390/md22100465
Novel Insights into Ethanol-Soluble Oyster Peptide-Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells
Abstract
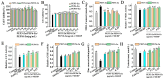
Numerous studies have reported that mono-(2-ethylhexyl) phthalate (MEHP) (bioactive metabolite of Di(2-ethylhexyl) phthalate) has inhibitory effects on Leydig cells. This study aims to prepare an oyster peptide-zinc complex (PEP-Zn) to alleviate MEHP-induced damage in Leydig cells. Zinc-binding peptides were obtained through the following processes: zinc-immobilized affinity chromatography (IMAC-Zn2+), liquid chromatography-mass spectrometry technology (LC-MS/MS) analysis, molecular docking, molecular dynamic simulation, and structural characterization. Then, the Zn-binding peptide (PEP) named Glu-His-Ala-Pro-Asn-His-Asp-Asn-Pro-Gly-Asp-Leu (EHAPNHDNPGDL) was identified. EHAPNHDNPGDL showed the highest zinc-chelating ability of 49.74 ± 1.44%, which was higher than that of the ethanol-soluble oyster peptides (27.50 ± 0.41%). In the EHAPNHDNPGDL-Zn complex, Asn-5, Asp-7, Asn-8, His-2, and Asp-11 played an important role in binding to the zinc ion. Additionally, EHAPNHDNPGDL-Zn was found to increase the cell viability, significantly increase the relative activity of antioxidant enzymes and testosterone content, and decrease malondialdehyde (MDA) content in MEHP-induced TM3 cells. The results also indicated that EHAPNHDNPGDL-Zn could alleviate MEHP-induced apoptosis by reducing the protein level of p53, p21, and Bax, and increasing the protein level of Bcl-2. These results indicate that the zinc-chelating peptides derived from oyster peptides could be used as a potential dietary zinc supplement.
Keywords: MEHP; TM3; apoptosis; in silico screening; zinc-chelating peptides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Xu J., Wang L., Zhang L., Zheng F., Wang F., Leng J., Wang K., Héroux P., Shen H.M., Wu Y., et al. Mono-2-ethylhexyl phthalate drives progression of PINK1- parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate. Redox Biol. 2021;38:101776. doi: 10.1016/j.redox.2020.101776. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

