Discovery of Anti-Inflammatory Alkaloids from Sponge Stylissa massa Suggests New Biosynthetic Pathways for Pyrrole-Imidazole Alkaloids
- PMID: 39452885
- PMCID: PMC11509139
- DOI: 10.3390/md22100477
Discovery of Anti-Inflammatory Alkaloids from Sponge Stylissa massa Suggests New Biosynthetic Pathways for Pyrrole-Imidazole Alkaloids
Abstract
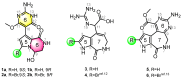
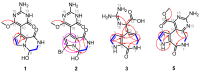
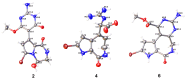
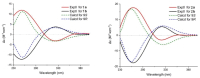
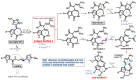
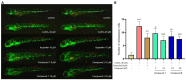
Pyrrole-imidazole alkaloids (PIAs) are a class of marine sponge derived natural products which have complex carbon frameworks and broad bioactivities. In this study, four new alkaloids, stylimassalins A-B (1-2), 3, and 5, together with two known compounds (4 and 6), were isolated from Stylissa massa. Compounds 2, 4, and 6 are the C-2 brominated analogues of 1, 3, and 5, respectively. Their structures display three different scaffolds, of which scaffold 1 (compounds 1,2) is new. A new biosynthetic pathway from oroidin, through spongiacidin, to latonduine and scaffold 1 was proposed by our group, in which the C12-N13-cleavaged compounds of spongiacidin (scaffold 2), dubbed seco-spongiacidins (3 and 4), are recognized as a key bridged scaffold, to afford PIA analogues (1,2 and 5,6). An anti-inflammatory evaluation in a zebrafish inflammation model induced by copper sulphate (CuSO4) demonstrated that stylimassalins A and B (1 and 2) could serve as a promising lead scaffold for treating inflammation.
Keywords: Stylissa massa; anti-inflammatory; biosynthetic pathway; latonduine; pyrrole–imidazole alkaloids; seco-spongiacidin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sharma G.M., Burkholder P.R. Structure of dibromophakellin, a new bromine-containing alkaloid from the marine sponge Phakellia flabellata. Chem. Commun. 1971;3:151–152. doi: 10.1039/c29710000151. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

