Metabolite Measurement in Index Substrate Drug Interaction Studies: A Review of the Literature and Recent New Drug Application Reviews
- PMID: 39452902
- PMCID: PMC11509402
- DOI: 10.3390/metabo14100522
Metabolite Measurement in Index Substrate Drug Interaction Studies: A Review of the Literature and Recent New Drug Application Reviews
Abstract
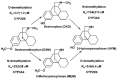
Background/objectives: Index substrates are used to understand the processes involved in pharmacokinetic (PK) drug-drug interactions (DDIs). The aim of this analysis is to review metabolite measurement in clinical DDI studies, focusing on index substrates for cytochrome P450 (CYP) enzymes, including CYP1A2 (caffeine), CYP2B6 (bupropion), CYP2C8 (repaglinide), CYP2C9 ((S)-warfarin, flurbiprofen), CYP2C19 (omeprazole), CYP2D6 (desipramine, dextromethorphan, nebivolol), and CYP3A (midazolam, triazolam).
Methods: All data used in this evaluation were obtained from the Certara Drug Interaction Database. Clinical index substrate DDI studies with PK data for at least one metabolite, available from literature and recent new drug application reviews, were reviewed. Further, for positive DDI studies, a correlation analysis was performed between changes in plasma exposure of index substrates and their marker metabolites.
Results: A total of 3261 individual index DDI studies were available, with 45% measuring at least one metabolite. The occurrence of metabolite measurement in clinical DDI studies varied widely between index substrates and enzymes.
Discussion and conclusions: For substrates such as caffeine, bupropion, omeprazole, and dextromethorphan, the use of the metabolite/parent area under the curve ratio can provide greater sensitivity to DDI or reduce intrasubject variability. In some cases (e.g., omeprazole, repaglinide), the inclusion of metabolite measurement can provide mechanistic insights to understand complex interactions.
Keywords: drug interaction; metabolism; metabolite; pharmacokinetics.
Conflict of interest statement
Jingjing Yu, Nathalie Rioux, Iain Gardner, Katie Owens, and Isabelle Ragueneau-Majlessi are employees of Certara. The paper reflects the views of the scientists and not the company.
Figures











References
-
- ICH Harmonised Guideline on Drug Interaction Studies M12. [(accessed on 1 June 2024)]. Available online: https://database.ich.org/sites/default/files/ICH_M12_Step4_Guideline_202....
Publication types
LinkOut - more resources
Full Text Sources

