Causal Metabolomic and Lipidomic Analysis of Circulating Plasma Metabolites in Autism: A Comprehensive Mendelian Randomization Study with Independent Cohort Validation
- PMID: 39452938
- PMCID: PMC11509474
- DOI: 10.3390/metabo14100557
Causal Metabolomic and Lipidomic Analysis of Circulating Plasma Metabolites in Autism: A Comprehensive Mendelian Randomization Study with Independent Cohort Validation
Abstract
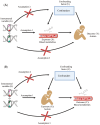
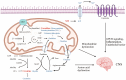
Background: The increasing prevalence of autism spectrum disorder (ASD) highlights the need for objective diagnostic markers and a better understanding of its pathogenesis. Metabolic differences have been observed between individuals with and without ASD, but their causal relevance remains unclear.
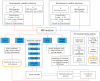
Methods: Bidirectional two-sample Mendelian randomization (MR) was used to assess causal associations between circulating plasma metabolites and ASD using large-scale genome-wide association study (GWAS) datasets-comprising 1091 metabolites, 309 ratios, and 179 lipids-and three European autism datasets (PGC 2015: n = 10,610 and 10,263; 2017: n = 46,351). Inverse-variance weighted (IVW) and weighted median methods were employed, along with robust sensitivity and power analyses followed by independent cohort validation.
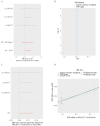
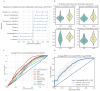
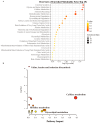
Results: Higher genetically predicted levels of sphingomyelin (SM) (d17:1/16:0) (OR, 1.129; 95% CI, 1.024-1.245; p = 0.015) were causally linked to increased ASD risk. Additionally, ASD children had higher plasma creatine/carnitine ratios. These MR findings were validated in an independent US autism cohort using machine learning analysis.
Conclusion: Utilizing large datasets, two MR approaches, robust sensitivity analyses, and independent validation, our novel findings provide evidence for the potential roles of metabolomics and circulating metabolites in ASD diagnosis and etiology.
Keywords: Mendelian randomization; autism spectrum disorder; causal inference; cohort validation; machine learning; metabolites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Myers S.M., Voigt R.G., Colligan R.C., Weaver A.L., Storlie C.B., Stoeckel R.E., Port J.D., Katusic S.K. Autism Spectrum Disorder: Incidence and Time Trends Over Two Decades in a Population-Based Birth Cohort. J. Autism Dev. Disord. 2019;49:1455–1474. doi: 10.1007/s10803-018-3834-0. - DOI - PMC - PubMed
-
- Hyman S.L., Levy S.E., Myers S.M., Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Kuo D.Z., Apkon S., Davidson L.F., Ellerbeck K.A., Foster J.E., Noritz G.H., et al. Identification, Evaluation, and Management of Children with Autism Spectrum Disorder. Pediatrics. 2020;145:e20193447. doi: 10.1542/peds.2019-3447. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

