Studying the Defects in Spinel Compounds: Discovery, Formation Mechanisms, Classification, and Influence on Catalytic Properties
- PMID: 39452977
- PMCID: PMC11510202
- DOI: 10.3390/nano14201640
Studying the Defects in Spinel Compounds: Discovery, Formation Mechanisms, Classification, and Influence on Catalytic Properties
Abstract
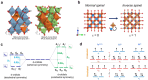
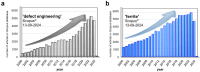
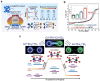
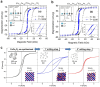
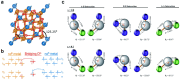
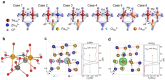
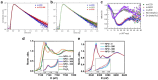
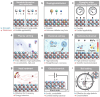
Spinel ferrites demonstrate extensive applications in different areas, like electrodes for electrochemical devices, gas sensors, catalysts, and magnetic adsorbents for environmentally important processes. However, defects in the real spinel structure can change the many physical and chemical properties of spinel ferrites. Although the number of defects in a crystal spinel lattice is small, their influence on the vast majority of physical properties could be really decisive. This review provides an overview of the structural characteristics of spinel compounds (e.g., CoFe2O4, NiFe2O4, ZnFe2O4, Fe3O4, γ-Fe2O3, Co3O4, Mn3O4, NiCo2O4, ZnCo2O4, Co2MnO4, etc.) and examines the influence of defects on their properties. Attention was paid to the classification (0D, 1D, 2D, and 3D defects), nomenclature, and the formation of point and surface defects in ferrites. An in-depth description of the defects responsible for the physicochemical properties and the methodologies employed for their determination are presented. DFT as the most common simulation approach is described in relation to modeling the point defects in spinel compounds. The significant influence of defect distribution on the magnetic interactions between cations, enhancing magnetic properties, is highlighted. The main defect-engineering strategies (direct synthesis and post-treatment) are described. An antistructural notation of active centers in spinel cobalt ferrite is presented. It is shown that the introduction of cations with different charges (e.g., Cu(I), Mn(II), Ce(III), or Ce(IV)) into the cobalt ferrite spinel matrix results in the formation of various point defects. The ability to predict the type of defects and their impact on material properties is the basis of defect engineering, which is currently an extremely promising direction in modern materials science.
Keywords: defect; ferrite; magnetism; spinel; structure; vacancy.
Conflict of interest statement
The author declares no conflicts of interest.
Figures








References
-
- Khan H., Naskar A., Bera S. 3—Vacancy and Defect Structures in Metal Oxides. In: Kumar V., Som S., Sharma V., Swart H.C.B.T.-M.O.D., editors. Metal Oxides. Elsevier; Amsterdam, The Netherlands: 2023. pp. 61–81.
-
- Gao S., Zhao S., Tang X., Sun L., Li Q., Yi H. Research on the Application of Defect Engineering in the Field of Environmental Catalysis. Green Energy Environ. 2024 doi: 10.1016/j.gee.2024.08.008. - DOI
-
- Hamed Mashhadzadeh A., Salmankhani A., Zarghami Dehghani M., Spitas C., Saeb M.R. 9—Influence of Defects upon Mechanical Properties of Oxide Materials. In: Kumar V., Som S., Sharma V., Swart H.C., editors. Metal Oxides. Elsevier; Amsterdam, The Netherlands: 2023. pp. 253–280.
-
- Shluger A. In: Defects in Oxides in Electronic Devices BT—Handbook of Materials Modeling: Applications: Current and Emerging Materials. Andreoni W., Yip S., editors. Springer International Publishing; Cham, Germany: 2018. pp. 1–22.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

