Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer
- PMID: 39453034
- PMCID: PMC11510840
- DOI: 10.3390/tomography10100117
Diagnostic Value of Contrast-Enhanced Dual-Energy Computed Tomography in the Pancreatic Parenchymal and Delayed Phases for Pancreatic Cancer
Abstract
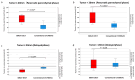
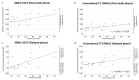
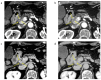
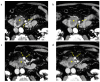
Background/Objectives: The usefulness of dual-energy computed tomography (DECT) for low absorption in the parenchymal phase and contrast effects in the delayed phase for pancreatic cancer is not clear. Therefore, the diagnostic capability of low-KeV images obtained using DECT for pancreatic cancer in the pancreatic parenchymal and delayed phases was evaluated quantitatively and qualitatively. Methods: Twenty-five patients with pancreatic cancer who underwent contrast-enhanced DECT were included. A total of 50 and 70 KeV CT images, classified as low-keV and conventional CT-equivalent images, were produced, respectively. The tumor-to-pancreas contrast (Hounsfield units [HU]) in the pancreatic parenchymal and delayed phases was calculated by subtracting the CT value of the pancreatic tumor from that of normal parenchyma. Results: The median tumor-to-pancreas contrast on 50 KeV CT in the pancreatic parenchymal phase (133 HU) was higher than that on conventional CT (68 HU) (p < 0.001). The median tumor-to-pancreas contrast in the delayed phase was -28 HU for 50 KeV CT and -9 HU for conventional CT (p = 0.545). For tumors < 20 mm, the tumor-to-pancreas contrast of 50 KeV CT (-39 HU) had a significantly clearer contrast effect than that of conventional CT (-16.5 HU), even in the delayed phase (p = 0.034). Conclusions: These 50 KeV CT images may clarify the low-absorption areas of pancreatic cancer in the pancreatic parenchymal phase. A good contrast effect was observed in small pancreatic cancers on 50 KeV delayed-phase images, suggesting that DECT is useful for the visualization of early pancreatic cancer with a small tumor diameter.
Keywords: contrast; delayed phase; dual-energy computed tomography; pancreatic cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

